H - Bruder Chemistry
... calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. ...
... calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. ...
Lecture 5
... • Since Gibbs Free Energy is defined as: G = H-TS dG = dH-TdS-SdT • For a reaction at constant temperature ∆G = ∆H-T∆S • Equilibrium states are characterized by minimum energy and maximum entropy. The Gibbs Free Energy is a function that decreases with decreasing energy (∆H) and increasing entropy ( ...
... • Since Gibbs Free Energy is defined as: G = H-TS dG = dH-TdS-SdT • For a reaction at constant temperature ∆G = ∆H-T∆S • Equilibrium states are characterized by minimum energy and maximum entropy. The Gibbs Free Energy is a function that decreases with decreasing energy (∆H) and increasing entropy ( ...
PPT
... It will “heat up” the system (i.e., raise T). It can make the system do work on the surroundings. Heat capacity is defined to be the heat required to raise the temperature of a system by 1K (=1º C). Its SI units are J/K. C ...
... It will “heat up” the system (i.e., raise T). It can make the system do work on the surroundings. Heat capacity is defined to be the heat required to raise the temperature of a system by 1K (=1º C). Its SI units are J/K. C ...
Traditional versus PIN Diode Geiger Counter
... sources is able to break out electrons from specific materials that can be detected as a current. The resulting electrical energy of the emitted electrons is equivalent to the energy of the radiation quants reduced by the material’s specific electron binding energy. A silicon photo or solar cell use ...
... sources is able to break out electrons from specific materials that can be detected as a current. The resulting electrical energy of the emitted electrons is equivalent to the energy of the radiation quants reduced by the material’s specific electron binding energy. A silicon photo or solar cell use ...
Chapter 9: Chemical Bonding I: Lewis Theory
... D) Found as a 3-D crystal lattices containing alternating cations & anions. 2) Covalent Bonding A) Covalent Bonding results from sharing valence electrons. B) Occurs between nonmetals. C) Most important kind of bond in chemistry. 3) Metallic Bonding a) Occurs in metals. b) Electron Sea Model: we wil ...
... D) Found as a 3-D crystal lattices containing alternating cations & anions. 2) Covalent Bonding A) Covalent Bonding results from sharing valence electrons. B) Occurs between nonmetals. C) Most important kind of bond in chemistry. 3) Metallic Bonding a) Occurs in metals. b) Electron Sea Model: we wil ...
file
... –Release of energy by the breakdown of complex molecules to simpler compounds –Breaking!! ...
... –Release of energy by the breakdown of complex molecules to simpler compounds –Breaking!! ...
Lecture 6/7 - TCD Chemistry
... J K-1g-1 or the molar heat capacity Cm as Cm = C/n with units: J K-1mol-1. The heat capacity depends on whether a sample is maintained at constant volume (C = CV) or constant pressure (C = CP). The respective molar quantities are CV,m and CP,m. Loss of energy into the surroundings can be detected by ...
... J K-1g-1 or the molar heat capacity Cm as Cm = C/n with units: J K-1mol-1. The heat capacity depends on whether a sample is maintained at constant volume (C = CV) or constant pressure (C = CP). The respective molar quantities are CV,m and CP,m. Loss of energy into the surroundings can be detected by ...
(Maximum 6 pages, including figures, tables and references, please
... the energy consumption of primary plant equipment. In particular, different envelope solutions in terms of wall layers were digitally implemented, and the results of the corresponding thermal simulations were compared. This allowed to identify efficient design choices from an energy point of view an ...
... the energy consumption of primary plant equipment. In particular, different envelope solutions in terms of wall layers were digitally implemented, and the results of the corresponding thermal simulations were compared. This allowed to identify efficient design choices from an energy point of view an ...
Calculations Formulas Definitions
... First Law: The mass of a substance reacting at the electrodes is directly proportional to the quantity of electricity passed through the solution. Second Law: The masses of different substances produced during electrolysis are directly proportional to their equivalent weights; 96,496 coulombs of ele ...
... First Law: The mass of a substance reacting at the electrodes is directly proportional to the quantity of electricity passed through the solution. Second Law: The masses of different substances produced during electrolysis are directly proportional to their equivalent weights; 96,496 coulombs of ele ...
Figure 4 - University of Wisconsin–Madison
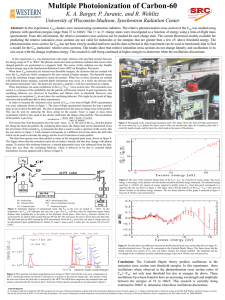
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
... photoionization cross section for C60 has not been closely studied until now and the data collected in this experiment can be used as benchmark data to find a model for the C60 molecules’ relative cross sections. The results show that relative ionization cross sections do not change linearly, and os ...
Electrons and “holes”
... The conductivity of intrinsic semiconductors can be due to crystal defects or to thermal excitation. In an intrinsic semiconductor the number of electrons in the conduction band is equal to the number of holes in the valence band. An example is Hg0.8Cd0.2Te at room temperature. An indirect gap intri ...
... The conductivity of intrinsic semiconductors can be due to crystal defects or to thermal excitation. In an intrinsic semiconductor the number of electrons in the conduction band is equal to the number of holes in the valence band. An example is Hg0.8Cd0.2Te at room temperature. An indirect gap intri ...
Chapter 6 Chemical Bonding
... How does covalent and ionic bonding differ at the atomic level? Ionic bonding: An e- is actually transferred from one atom to the other This causes the donator to shrink and the acceptor to enlarge The donator becomes (+) and the acceptor (-) Structure is held together because of opposites a ...
... How does covalent and ionic bonding differ at the atomic level? Ionic bonding: An e- is actually transferred from one atom to the other This causes the donator to shrink and the acceptor to enlarge The donator becomes (+) and the acceptor (-) Structure is held together because of opposites a ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.