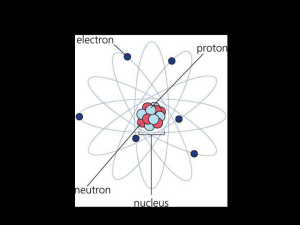
All substances are made from atoms
... smallest particle which exists of an element. All of the atoms of any one element (say oxygen) are identical. Oxygen gas is made from trillions of identical oxygen atoms. There are just over one hundred elements in the periodic table, so there are just over one hundred types of atoms in the universe ...
... smallest particle which exists of an element. All of the atoms of any one element (say oxygen) are identical. Oxygen gas is made from trillions of identical oxygen atoms. There are just over one hundred elements in the periodic table, so there are just over one hundred types of atoms in the universe ...
The atomic number tells how many protons Protons make an atom
... p+ and no, but some rare atoms will have only 196. They ALL have 79 p+. Most have 118no, but a few may have 117 no. ...
... p+ and no, but some rare atoms will have only 196. They ALL have 79 p+. Most have 118no, but a few may have 117 no. ...
Introductory Chemistry
... Based on the American convention, Roman numeral indicates the number of valence electrons. – Group IA elements have: 1 valence electron – Group VA elements have: 5 valence electrons Based on the IUPAC designations for group numbers, the last digit indicates the number of valence electrons. – Group 1 ...
... Based on the American convention, Roman numeral indicates the number of valence electrons. – Group IA elements have: 1 valence electron – Group VA elements have: 5 valence electrons Based on the IUPAC designations for group numbers, the last digit indicates the number of valence electrons. – Group 1 ...
ScienceHelpNotes-UnitB3 - JA Williams High School
... into the periodic table. The periodic table is separated by a staircase, with metals on the left of the staircase and nonmetals on the right. Metals have some similarities in their chemical properties because they all lose electrons to form positive ions. Typically, metal are shiny, flexible, and ...
... into the periodic table. The periodic table is separated by a staircase, with metals on the left of the staircase and nonmetals on the right. Metals have some similarities in their chemical properties because they all lose electrons to form positive ions. Typically, metal are shiny, flexible, and ...
Atoms: The Building Blocks of Matter
... take a pair of shears and cut a piece of copper in two and sometime, you would reach a piece that couldn’t be cut anymore. He named this particle an atom meaning indivisible. ...
... take a pair of shears and cut a piece of copper in two and sometime, you would reach a piece that couldn’t be cut anymore. He named this particle an atom meaning indivisible. ...
Chapter 3: Atoms and Moles By: John Pierce
... the mass of the products. The law of multiple proportions states that when two elements merge to create two or more compounds, the mass of one element that combines with a given mass of the other is in the ratio of small whole numbers. ...
... the mass of the products. The law of multiple proportions states that when two elements merge to create two or more compounds, the mass of one element that combines with a given mass of the other is in the ratio of small whole numbers. ...
Atoms, Molecules and Ions Part 2
... Isotopes • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms o ...
... Isotopes • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms o ...
Document
... Some Properties of Nuclei The atomic number, Z, equals the number of protons in the nucleus. The neutron number, N, is the number of neutrons in the nucleus. The mass number, A, is the number of nucleons in the nucleus. A=Z+N “Nucleon” is a generic term used to refer to either a proton or a neutron ...
... Some Properties of Nuclei The atomic number, Z, equals the number of protons in the nucleus. The neutron number, N, is the number of neutrons in the nucleus. The mass number, A, is the number of nucleons in the nucleus. A=Z+N “Nucleon” is a generic term used to refer to either a proton or a neutron ...
notes - unit 3 - periodic table_student_2014
... _________________________________________________ _________________________________________________ 7. Periods are similar to what on a calendar? _________________ 8. Groups are similar to what on a calendar? __________________ 9. Use the calendar analogy to explain whether it’s more likely to find ...
... _________________________________________________ _________________________________________________ 7. Periods are similar to what on a calendar? _________________ 8. Groups are similar to what on a calendar? __________________ 9. Use the calendar analogy to explain whether it’s more likely to find ...
File - Flipped Out Science with Mrs. Thomas!
... Atom – The atom is a basic unit of matter and the smallest unit of an element. Atomic Mass – The total number of protons and neutrons in an atom Atomic Number – The number of protons in an atomic nucleus Balanced equation - occurs when the number of the different atoms of elements in the reactants s ...
... Atom – The atom is a basic unit of matter and the smallest unit of an element. Atomic Mass – The total number of protons and neutrons in an atom Atomic Number – The number of protons in an atomic nucleus Balanced equation - occurs when the number of the different atoms of elements in the reactants s ...
File - Flipped Out Science with Mrs. Thomas!
... Atom – The atom is a basic unit of matter and the smallest unit of an element. Atomic Mass – The total number of protons and neutrons in an atom Atomic Number – The number of protons in an atomic nucleus Balanced equation - occurs when the number of the different atoms of elements in the reactants s ...
... Atom – The atom is a basic unit of matter and the smallest unit of an element. Atomic Mass – The total number of protons and neutrons in an atom Atomic Number – The number of protons in an atomic nucleus Balanced equation - occurs when the number of the different atoms of elements in the reactants s ...
Atomic Theory Powerpoint
... Atoms are indivisible and indestructible Did not explain chemical behavior and ...
... Atoms are indivisible and indestructible Did not explain chemical behavior and ...
Chem 1151
... The electron configuration [Kr] describes the electron configuration for all of the following except A. **B. C. D. ...
... The electron configuration [Kr] describes the electron configuration for all of the following except A. **B. C. D. ...
Topic 5: The periodic Table
... • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The term “chemical family” describes a group of rela ...
... • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The term “chemical family” describes a group of rela ...
Uint one - pisscience
... *He classified each main group into two subgroups (A,B) because he found differences between their properties. Advantages of Mendeleev's Table: 1-He left spaces in his table as he predicted the discovery of new elements. 2-He corrected the wrong estimated atomic weights of some elements. Disadvantag ...
... *He classified each main group into two subgroups (A,B) because he found differences between their properties. Advantages of Mendeleev's Table: 1-He left spaces in his table as he predicted the discovery of new elements. 2-He corrected the wrong estimated atomic weights of some elements. Disadvantag ...
DO NOW - PBworks
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
The Periodic Table
... cannot be split into smaller particles Atoms cannot be created or destroyed All atoms of the same element have the same properties, and the atoms of different elements have different properties Atoms of different elements can combine to form new substances. ...
... cannot be split into smaller particles Atoms cannot be created or destroyed All atoms of the same element have the same properties, and the atoms of different elements have different properties Atoms of different elements can combine to form new substances. ...
Atomic Theory
... __________ _________, similarities in their properties occur in a regular pattern. a) Atomic mass b) Atomic number c) Atomic radius ...
... __________ _________, similarities in their properties occur in a regular pattern. a) Atomic mass b) Atomic number c) Atomic radius ...
Chapter 4.1 and 4.2 - science-b
... part, but the rest of this tenet is still fundamental to chemistry. ...
... part, but the rest of this tenet is still fundamental to chemistry. ...
Isotopes
... Bohr Model • This restricts electrons to certain orbits and cannot effectively model all the elements [http://www.incompetech.com/gallimaufry/signs/bohr.gif] ...
... Bohr Model • This restricts electrons to certain orbits and cannot effectively model all the elements [http://www.incompetech.com/gallimaufry/signs/bohr.gif] ...
Multiple choice questions
... 2. Examine the points made by Dalton in his atomic theory. Consider each statement, and record: (a) whether or not each suggestion still holds (b) which other scientists were able to contribute to refining or changing each proposal, and in what way (c) our current understanding of each statement. 3. ...
... 2. Examine the points made by Dalton in his atomic theory. Consider each statement, and record: (a) whether or not each suggestion still holds (b) which other scientists were able to contribute to refining or changing each proposal, and in what way (c) our current understanding of each statement. 3. ...
Review Sheet for Chemistry* First Semester Final
... Electron Configurations. What element has the configuration [Ne]3s23p1? _____ What does the 3 mean in 3s2 ? ...
... Electron Configurations. What element has the configuration [Ne]3s23p1? _____ What does the 3 mean in 3s2 ? ...
Matter and Chemical Change PPT
... changed, but not its chemical composition. The change is temporary/reversible. E.g. ice melts to form a puddle of water, we dissolve sugar in water. Chemical Change: causes one or more new substances, with new properties, to be formed and may be difficult or impossible to reverse. e.g. burning paper ...
... changed, but not its chemical composition. The change is temporary/reversible. E.g. ice melts to form a puddle of water, we dissolve sugar in water. Chemical Change: causes one or more new substances, with new properties, to be formed and may be difficult or impossible to reverse. e.g. burning paper ...
Chapter 4 Section 4.1 & 4.2
... Dalton’s Atomic Theory 4. Chemical reactions occur when atoms are separated from each other, joined, or rearranged in different combinations. Atoms of one element are never changed into atoms of another element as a result of a chemical reaction. Compound made by chemically combining atoms of eleme ...
... Dalton’s Atomic Theory 4. Chemical reactions occur when atoms are separated from each other, joined, or rearranged in different combinations. Atoms of one element are never changed into atoms of another element as a result of a chemical reaction. Compound made by chemically combining atoms of eleme ...