Problem Set 5_Chem165_Spring14
... However, I don’t think there’s cause for panic. The tanks have been there a long time and there have been a lot of small leaks. That’s not good, and it needs to be fixed. But the cleanup needs to be done carefully and thoughtfully. The materials is unbelievably hazardous and corrosive, so the proces ...
... However, I don’t think there’s cause for panic. The tanks have been there a long time and there have been a lot of small leaks. That’s not good, and it needs to be fixed. But the cleanup needs to be done carefully and thoughtfully. The materials is unbelievably hazardous and corrosive, so the proces ...
General Properties of Transition Metals
... • Transition metals can form complexes because their ions have a high charge density: o they have quite a large nuclear charge but are relatively small; o the 3d electrons are not so effective (as 2s or 2p electrons) at shielding the effect of the ionic charge which really comes from the nucleus. ...
... • Transition metals can form complexes because their ions have a high charge density: o they have quite a large nuclear charge but are relatively small; o the 3d electrons are not so effective (as 2s or 2p electrons) at shielding the effect of the ionic charge which really comes from the nucleus. ...
Chapter 19 d-Metal complexes: electronic structure and spectra
... electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was ...
... electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes, but it does not attempt to describe bonding. CFT was ...
ElectronicStructureSurfaces.pdf
... we must consider the structure of the solid as a whole. This provides the basis for the description of metals and other types of solids to account for their unique chemical and physical properties. To fully understand the properties,it is essential to start with molecular orbital theory. In the basi ...
... we must consider the structure of the solid as a whole. This provides the basis for the description of metals and other types of solids to account for their unique chemical and physical properties. To fully understand the properties,it is essential to start with molecular orbital theory. In the basi ...
Practice Test
... (D) Iodine liberates free bromine from a solution of bromide ion. (E) Fluorine is the most electronegative of the halogens. ...
... (D) Iodine liberates free bromine from a solution of bromide ion. (E) Fluorine is the most electronegative of the halogens. ...
Final Exam Review
... E. shiny 31. In general, how are metalloids different from metals and nonmetals? 51. In which pair of elements are the chemical properties of the elements most similar? Explain your reasoning. A. sodium and chlorine B. nitrogen and phosphorous C. boron and oxygen 88. The volume of a liquid in a grad ...
... E. shiny 31. In general, how are metalloids different from metals and nonmetals? 51. In which pair of elements are the chemical properties of the elements most similar? Explain your reasoning. A. sodium and chlorine B. nitrogen and phosphorous C. boron and oxygen 88. The volume of a liquid in a grad ...
Polydentate ligand (macrocycle) bound to Fe via 4 N atoms. This is
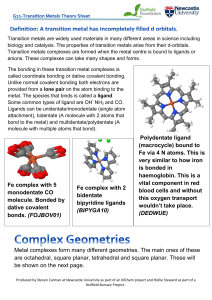
... Transition metals are widely used materials in many different areas in science including biology and catalysis. The properties of transition metals arise from their d-orbitals. Transition metals complexes are formed when the metal centre is bound to ligands or anions. These complexes can take many s ...
... Transition metals are widely used materials in many different areas in science including biology and catalysis. The properties of transition metals arise from their d-orbitals. Transition metals complexes are formed when the metal centre is bound to ligands or anions. These complexes can take many s ...
Spin states (d electrons)
... the metal center, the charge of the metal center, and the field strength of the complex's ligands as described by the spectrochemical series. In order for low spin splitting to occur, the energy cost Low-spin [Fe(NO2)6]3− crystal field diagram of placing an electron into an already singly occupied o ...
... the metal center, the charge of the metal center, and the field strength of the complex's ligands as described by the spectrochemical series. In order for low spin splitting to occur, the energy cost Low-spin [Fe(NO2)6]3− crystal field diagram of placing an electron into an already singly occupied o ...
Slide 1
... – The final -e is dropped from the name of the parent hydrocarbon – The suffix -oic is added followed by the word acid. Many organic acids are called by their common (trivial) names which are derived from Greek or Latin. ...
... – The final -e is dropped from the name of the parent hydrocarbon – The suffix -oic is added followed by the word acid. Many organic acids are called by their common (trivial) names which are derived from Greek or Latin. ...
Absorption Spectra and Colours of Complexes
... 3. PPh3 < CN- < CO. It is surely astonishing to many chemists that not only do carbon monoxide and phosphine ligands bond readily to many transition metals, but that of all the ligands, they (together with cyanide) have the greatest capacity to split the d-orbitals. Let's consider what happens when ...
... 3. PPh3 < CN- < CO. It is surely astonishing to many chemists that not only do carbon monoxide and phosphine ligands bond readily to many transition metals, but that of all the ligands, they (together with cyanide) have the greatest capacity to split the d-orbitals. Let's consider what happens when ...
18 Valence Electron Rule
... compounds. Owing to small Δ tetr splitting energy, the tetrahedral transition metal complexes also belongs to this class. Class II: In class II complexes, the Δ o splitting is relatively large and is applicable to 4d and 5d transition metals having high oxidation state and for σ ligands in the inte ...
... compounds. Owing to small Δ tetr splitting energy, the tetrahedral transition metal complexes also belongs to this class. Class II: In class II complexes, the Δ o splitting is relatively large and is applicable to 4d and 5d transition metals having high oxidation state and for σ ligands in the inte ...
Chapter 4 Notes
... • Energies of atoms are fixed and definite quantities • Energy transitions occur in jumps of discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
... • Energies of atoms are fixed and definite quantities • Energy transitions occur in jumps of discrete amounts of energy • Electrons only lose energy when they move to a lower energy state ...
23-24
... complexes, magnetic behavior. Ligand Field Theory (LFT) is much simpler than MO theory (a little more sophisticated than CFT), but it is a very useful theory. ...
... complexes, magnetic behavior. Ligand Field Theory (LFT) is much simpler than MO theory (a little more sophisticated than CFT), but it is a very useful theory. ...
Practice final
... 22. Which of the following statements is/are TRUE ? A. The wavefunction, ψ, is a solution to the Schrodinger equation B. The square of the wavefunction, ψ 2, is the total probability of finding the electron in a spherical shell at distance r from the nucleus C. Orbitals with the same n cannot overla ...
... 22. Which of the following statements is/are TRUE ? A. The wavefunction, ψ, is a solution to the Schrodinger equation B. The square of the wavefunction, ψ 2, is the total probability of finding the electron in a spherical shell at distance r from the nucleus C. Orbitals with the same n cannot overla ...
Slide 1
... – The final -e is dropped from the name of the parent hydrocarbon – The suffix -oic is added followed by the word acid. Many organic acids are called by their common (trivial) names which are derived from Greek or Latin. ...
... – The final -e is dropped from the name of the parent hydrocarbon – The suffix -oic is added followed by the word acid. Many organic acids are called by their common (trivial) names which are derived from Greek or Latin. ...
1 0 +1
... constructed. The table is a grid of all possible electronic arrangements. It lists all of the possible values of spin and orbital orientation. It includes both ground and excited states, and must obey the Pauli Exclusion Principle. ...
... constructed. The table is a grid of all possible electronic arrangements. It lists all of the possible values of spin and orbital orientation. It includes both ground and excited states, and must obey the Pauli Exclusion Principle. ...
Chapter 5
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
Review for second exam:
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
... valence electrons for main group elements alkali metals, alkali earth metals, halogens, noble gases metals, nonmetals, metalloids (semimetals); general properties and location Effective nuclear charge, Zeff; approximate value for Zeff, calculation and interpretation Zeff and Coulomb’s law Trends in ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 24. What is lanthanide contraction? What are its consequences? ...
... 24. What is lanthanide contraction? What are its consequences? ...
Coordination Chemistry: Bonding, Spectra, and Magnetism
... Recall that the dxy, dxz, dyz, and dx2-y2 orbitals all look the same because mathematically the only difference between them is their orientation in space. The dz2 orbital looks different because it is the average of the two remaining mathematical functions. The “doughnut” traditionally shown in gen ...
... Recall that the dxy, dxz, dyz, and dx2-y2 orbitals all look the same because mathematically the only difference between them is their orientation in space. The dz2 orbital looks different because it is the average of the two remaining mathematical functions. The “doughnut” traditionally shown in gen ...
Document
... bulkier P(t-Bu)3. Apparently this is even worse for the smaller Cr center (consistent with a steric argument) and explains the non-existence of that complex. An additional problem could be the generally weaker Cr-P (vs W-P) bonds, so that even a long Cr-P bond might be too weak to be profitable. c. ...
... bulkier P(t-Bu)3. Apparently this is even worse for the smaller Cr center (consistent with a steric argument) and explains the non-existence of that complex. An additional problem could be the generally weaker Cr-P (vs W-P) bonds, so that even a long Cr-P bond might be too weak to be profitable. c. ...
Write the symbols and electronic configurations for each of the first
... Scandium is not considered a transition element because it’s common oxidation state, +3, has no d block electrons. (Strictly speaking the metal is a transition element but the compounds with the Sc3+ ion, are not.) Typical (characteristic) properties of transition metals (d-block elements): Va ...
... Scandium is not considered a transition element because it’s common oxidation state, +3, has no d block electrons. (Strictly speaking the metal is a transition element but the compounds with the Sc3+ ion, are not.) Typical (characteristic) properties of transition metals (d-block elements): Va ...
Jahn–Teller effect
-3D-balls.png?width=300)
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbital nonlinear spatially degenerate molecules cannot be stable. The Jahn–Teller theorem essentially states that any nonlinear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geometrical distortion that occurs in crystals with substitutional impurities see article off-center ions.