Density of states
... with a low electron density and describe a charge-phonon coupling. There are two different kinds of polarons, the Fröhlich Polaron and the Holstein Polaron. The Fröhlich polarons describe large polarons, that means the distortion is much larger than the lattice constant of the material so that a l ...
... with a low electron density and describe a charge-phonon coupling. There are two different kinds of polarons, the Fröhlich Polaron and the Holstein Polaron. The Fröhlich polarons describe large polarons, that means the distortion is much larger than the lattice constant of the material so that a l ...
2A Final Exam Review Worksheet
... 20. Circle the correct answer in the table below. Question: Which element has the lower first ionization energy? Which orbital is better at shielding (screening)? The oxide of which element can react with both acids and bases? Which chemical species is a better reducing agent? Which bond is more cov ...
... 20. Circle the correct answer in the table below. Question: Which element has the lower first ionization energy? Which orbital is better at shielding (screening)? The oxide of which element can react with both acids and bases? Which chemical species is a better reducing agent? Which bond is more cov ...
Experimental study on transition metal complexes containing N,S
... distances indicate dianionic form of ligands leading to a spin triplet ground state for 6b. The DFT calculations on the similar compounds suggested two possibilities, namely: CoIII (d6), or CoII (d7) coordinated to a ligand radical, though the structural features of 6b point toward CoIII (d6) config ...
... distances indicate dianionic form of ligands leading to a spin triplet ground state for 6b. The DFT calculations on the similar compounds suggested two possibilities, namely: CoIII (d6), or CoII (d7) coordinated to a ligand radical, though the structural features of 6b point toward CoIII (d6) config ...
Lecture 4
... Overlap with 3d is small (bonding dominated by overlap with 4s) Overlap improves down group so bond strengths increase 3d < 4d < 5d Contrast main group where bond strengths decrease 2p < 3p < 4p < 5p ...
... Overlap with 3d is small (bonding dominated by overlap with 4s) Overlap improves down group so bond strengths increase 3d < 4d < 5d Contrast main group where bond strengths decrease 2p < 3p < 4p < 5p ...
Lecture 2
... or cations with d electrons not available for π-bonding Soft acids are cations with a moderate positive charge (2+ or lower), Or cations with d electrons readily availbale for π-bonding ...
... or cations with d electrons not available for π-bonding Soft acids are cations with a moderate positive charge (2+ or lower), Or cations with d electrons readily availbale for π-bonding ...
pdf notes
... Three orbitals (6 e-) act as non-bonding, so you can’t count those e-. Three orbitals (6 e-) are involved is “ex-cluster” bonding (i.e., holding the fragment together), so you can’t count those either: x = v + n –12, where x is the # of donor ev is the valence e- for the transition metal n is the va ...
... Three orbitals (6 e-) act as non-bonding, so you can’t count those e-. Three orbitals (6 e-) are involved is “ex-cluster” bonding (i.e., holding the fragment together), so you can’t count those either: x = v + n –12, where x is the # of donor ev is the valence e- for the transition metal n is the va ...
Table showing examples of Complex ions with their bond
... Ionisation Energy: Is the energy required to remove an electron in its ground state from an atom to infinity. Quantitatively measured in kJmol—1 M(g) = M+ (g) + e— H = kJmol— ( - Change in, H – energy). The first ionisation energy is the energy required to remove one electron from the parent atom. ...
... Ionisation Energy: Is the energy required to remove an electron in its ground state from an atom to infinity. Quantitatively measured in kJmol—1 M(g) = M+ (g) + e— H = kJmol— ( - Change in, H – energy). The first ionisation energy is the energy required to remove one electron from the parent atom. ...
as a PDF
... For open shell molecules the situation is rather more complicated but here too Koopmans' theorem is applicable in certain instances. Thus, it is possible, at least in principle, to obtain directly from experiment an orbital energy diagram for the molecule in question. In this lecture I shall first d ...
... For open shell molecules the situation is rather more complicated but here too Koopmans' theorem is applicable in certain instances. Thus, it is possible, at least in principle, to obtain directly from experiment an orbital energy diagram for the molecule in question. In this lecture I shall first d ...
Ferroelectrics from first principles Designing ferroelectrics
... complex is independent of ✓, which means that the p ground state correspondsdz2 dx2-y2 to any point on the circle of radius ⇢ = = g/ C, where C is the stiffness t2g constant associated with the vibrations. This situation corresponds to a dynamic coupling of the e electrons to the modes Q2 and Q3 and ...
... complex is independent of ✓, which means that the p ground state correspondsdz2 dx2-y2 to any point on the circle of radius ⇢ = = g/ C, where C is the stiffness t2g constant associated with the vibrations. This situation corresponds to a dynamic coupling of the e electrons to the modes Q2 and Q3 and ...
chemistry - cloudfront.net
... indicates the main energy level occupied by the electrons (n). How many electrons can occupy an s orbital, p orbital, d and f orbitals? S=2, p=6, d=10, f=14 Which atom would have an octet of electrons (full s and p orbitals): Ar (He only has 2 electrons) PERIODIC TABLE Who is Dmitri Mendeleev? ...
... indicates the main energy level occupied by the electrons (n). How many electrons can occupy an s orbital, p orbital, d and f orbitals? S=2, p=6, d=10, f=14 Which atom would have an octet of electrons (full s and p orbitals): Ar (He only has 2 electrons) PERIODIC TABLE Who is Dmitri Mendeleev? ...
CHEM1002 2014-N-3 November 2014 • Transition metals are often
... the Cl- ion which donates a lone pair to the Ni2+ ion to make the bond. This type of bond is sometimes called a ‘dative’ or ‘coordinate’ bond. What is a chelate complex? Some ligands have more than one atom with a lone pair and can bond to a metal ion more than once. An example of this type of ligan ...
... the Cl- ion which donates a lone pair to the Ni2+ ion to make the bond. This type of bond is sometimes called a ‘dative’ or ‘coordinate’ bond. What is a chelate complex? Some ligands have more than one atom with a lone pair and can bond to a metal ion more than once. An example of this type of ligan ...
Absorption Spectra and Electron-Vibration Coupling of Ti:Sapphire
... energy levels mainly involve the d orbitals of Ti dopant and p orbitals of the six neighboring O ions. Thus, the relative positions of the nearest O ions have the most important effect on the crystal field on the dopant. Based on these considerations, we assume that the lattice vibrational effects a ...
... energy levels mainly involve the d orbitals of Ti dopant and p orbitals of the six neighboring O ions. Thus, the relative positions of the nearest O ions have the most important effect on the crystal field on the dopant. Based on these considerations, we assume that the lattice vibrational effects a ...
Atoms and Elements
... putting electrons into orbitals that have the same energy as each other. Put one electron into each orbital before pairing them up. Whichever way the first arrow (electron) points, the others must point the same way until they pair up, then they point in opposite directions. ...
... putting electrons into orbitals that have the same energy as each other. Put one electron into each orbital before pairing them up. Whichever way the first arrow (electron) points, the others must point the same way until they pair up, then they point in opposite directions. ...
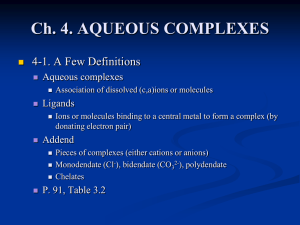
Ch. 3. KINETIC VS. EQUILIBRIUM MODELING
... Ions or molecules binding to a central metal to form a complex (by donating electron pair) ...
... Ions or molecules binding to a central metal to form a complex (by donating electron pair) ...
Lecture 12 Quantum Mechanics and Atomic Orbitals Bohr and
... properties of particles. λ = h/mv. However, these results only applied to systems with one electron. Attempts to apply these models to more complex atomic systems failed.In 1926, three scientists, Erwin Schrödinger, Werner Heisenberg and PaulDirac, introduced totally different approaches to the theo ...
... properties of particles. λ = h/mv. However, these results only applied to systems with one electron. Attempts to apply these models to more complex atomic systems failed.In 1926, three scientists, Erwin Schrödinger, Werner Heisenberg and PaulDirac, introduced totally different approaches to the theo ...
29th Annual Meeting | American Society of Preventive Oncology
... Our laboratory is involved in the development of safe organo-metallic complexes using natural biological molecules as ligands. These are nutritional supplements as well as candidate chemotherapy agents. The initial phase of development of these materials is based on the electronic signaling properti ...
... Our laboratory is involved in the development of safe organo-metallic complexes using natural biological molecules as ligands. These are nutritional supplements as well as candidate chemotherapy agents. The initial phase of development of these materials is based on the electronic signaling properti ...
PDF Version of Point Groups
... A contains three C2 axes, i.e., [Ck?] is yes with k=2. It contains a plane of symmetry so [σ?] is yes. The three C2 axes are perpendicular, i.e, there is a C2 axis and two perpendicular C2's which means that [⊥C2?] is yes. There is a plane of symmetry perpendicular to the C2 so [σh?] is yes and we a ...
... A contains three C2 axes, i.e., [Ck?] is yes with k=2. It contains a plane of symmetry so [σ?] is yes. The three C2 axes are perpendicular, i.e, there is a C2 axis and two perpendicular C2's which means that [⊥C2?] is yes. There is a plane of symmetry perpendicular to the C2 so [σh?] is yes and we a ...
Chemistry ~ Fall Final Review
... Bring a calculator & something to write with. You may bring a 4x6 note card w/ notes on both sides (MUST be handwritten) You will be expected to show all work, use correct significant figures and include proper units. ...
... Bring a calculator & something to write with. You may bring a 4x6 note card w/ notes on both sides (MUST be handwritten) You will be expected to show all work, use correct significant figures and include proper units. ...
P084
... oxide superlattices. While the bulk SrRuO$_{3}$ is well known to have a metallic ferromagnetic ground state, its surface electronic structure remains controversial as to possible changes of magnetic characteristics and metal-insulator transitions as well. We carried out firstprinciples local density ...
... oxide superlattices. While the bulk SrRuO$_{3}$ is well known to have a metallic ferromagnetic ground state, its surface electronic structure remains controversial as to possible changes of magnetic characteristics and metal-insulator transitions as well. We carried out firstprinciples local density ...
Strong electron correlations in cobalt valence tautomers
... ab initio calculations [11]. For the high-temperature geometry, the electronic structure calculation yields highspin (∼ 3/2) Co coupled ferromagnetically to spin 1/2 on each of the ligands. Furthermore we find that the low-spin configuration cannot be stabilized for the high-temperature geometry. In ...
... ab initio calculations [11]. For the high-temperature geometry, the electronic structure calculation yields highspin (∼ 3/2) Co coupled ferromagnetically to spin 1/2 on each of the ligands. Furthermore we find that the low-spin configuration cannot be stabilized for the high-temperature geometry. In ...
HMK Inorganic TutorialHMK
... (b) Since both nitrogen and boron have three bonding electrons, why do NH3 and BCl3 not have the same molecular shape? ...
... (b) Since both nitrogen and boron have three bonding electrons, why do NH3 and BCl3 not have the same molecular shape? ...
2010 Midterm 2 KEY
... The compound trans-Fe(o-phen)2(NCS)2 has a magnetic moment of 5.0 BM at room temperature but it drops steadily as the temperature is lowered so that it is only 0.65 by 80K and approaches 0.0 BM near 0K. (a) Calculate the approximate number of unpaired electrons in this complex at room temperature ba ...
... The compound trans-Fe(o-phen)2(NCS)2 has a magnetic moment of 5.0 BM at room temperature but it drops steadily as the temperature is lowered so that it is only 0.65 by 80K and approaches 0.0 BM near 0K. (a) Calculate the approximate number of unpaired electrons in this complex at room temperature ba ...
Chapter 4 - kkallenbach
... Photons – a quantum of light energy In order for the electron to be given off from the metal, the electron must be struck by a photon having enough energy to knock the electron loose ...
... Photons – a quantum of light energy In order for the electron to be given off from the metal, the electron must be struck by a photon having enough energy to knock the electron loose ...
Orbital orientation of the 4f ground state in CeCu2Si2 - SPring-8
... compounds is linked to the ground state wave function of the f electrons. In a crystalline environment an ion experiences a so-called crystalline electric field (CEF) caused by the charges of the surrounding ions and the degeneracy of the Hund’s rule ground state is lifted. The resulting CEF 4 f sta ...
... compounds is linked to the ground state wave function of the f electrons. In a crystalline environment an ion experiences a so-called crystalline electric field (CEF) caused by the charges of the surrounding ions and the degeneracy of the Hund’s rule ground state is lifted. The resulting CEF 4 f sta ...
Jahn–Teller effect
-3D-balls.png?width=300)
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that is associated with certain electron configurations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory, that orbital nonlinear spatially degenerate molecules cannot be stable. The Jahn–Teller theorem essentially states that any nonlinear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because the distortion lowers the overall energy of the species. For a description of another type of geometrical distortion that occurs in crystals with substitutional impurities see article off-center ions.