* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download General Properties of Transition Metals
Survey
Document related concepts
Transcript
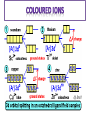
TRANSITION ELEMENTS: • Lie within the d-block elements • Titanium Copper • Metals Physical properties Conductors of heat & electricity Shiny, strong & hard High melting & boiling points Zinc is a d-block element but NOT transition element…you will see why later! GENERAL PROPERTIES • The general properties of transition metals are: Chemical properties – they form complexes – they form coloured ions – they form variable oxidation states – they have catalytic activity ELECTRONIC STRUCTURE • All characteristic properties are a result of their electronic structure • The transition metals have a partially filled 3d energy sub level in their atoms or ions • In general there are two outer 4s electrons, and electrons are added to the inner 3d sub level CHROMIUM & COPPER • In chromium the 4s13d5 structure is adopted because the repulsion between two paired electrons in the 4s orbital is more than the energy difference between the 4s and 3d subshells • It is therefore more stable to have unpaired electrons in the higher energy 3d orbital than paired electrons in the lower energy 4s orbital. • For copper it is more stable to have fully paired 3d shell. 1 electron drops from the 4s to achieve this and give 4s13d10 FORMING IONS • In all ions of d-block elements, the 3d subshell is lower in energy than the 4s subshell • So the 4s electrons are always removed first • 3d electrons are only removed after all 4s electrons have been removed • E.g. Fe = [Ar]3d64s2 Fe2+ = [Ar]3d6 • E.g. Fe2+ = [Ar]3d6 Fe3+ = [Ar]3d5 CONFIGURATION OF D-BLOCK IONS Give the electronic structure of the following ions: • Sc3+ 1s2 2s2 2p6 3s2 3p6 • Fe2+ 1s2 2s2 2p6 3s2 3p6 3d6 • Co3+ 1s2 2s2 2p6 3s2 3p6 3d6 • Cu+ 1s2 2s2 2p6 3s2 3p6 3d10 DEFINING A TRANSITION ELEMENT • A transition element is one that forms at least one stable ion with a part full d-shell of electrons • Zinc only forms Zn2+ (3d10) • It is a d-block elements and not a transition element Oxidation Numbers shown by first row d-block elements Sc Ti V Cr Mn Fe Co Ni Cu +I +I +I +I +I +I +I +I +I +II +II +II +II +II +II +II +II +II +III +III +III +III +III +III +III +III +III +IV +IV +IV +IV +IV +IV +IV +V +V +V +V +V +VI +VI +VI +VII Most common oxidation states highlighted (not all stable) Complete the sheet to assess your progress Zn +II ANSWERS: 1 a) [Ar]3d34s2 1 b) [Ar]3d74s2 1 c) [Ar]3d104s1 1 d) [Ar]3d84s2 2) Physical Properties – high density, high melting points, high boiling points, hard, shiny Chemical properties – form complex ions, form coloured ions, act as catalysts, exist in variable oxidation states ANSWERS: 3) A metal that can form one or more stable ions with a partially filled d-subshell 4) Electrons fill up the lowest energy subshells first. Electrons fill orbitals singly before sharing 5) Zinc has the configuration [Ar]3d104s2. It can only form Zn2+ ions ([Ar]3d10). This ion has a full 3d-subshell. Zinc cannot form a stable ion with a partially filled 3d-subshell so it is not a transition metal. ANSWERS: 6) Chromium prefers to have one electron in each orbital of the 3d subshell and just one in the 4s as this gives it more stability 7) Copper prefers to have a full 3d subshell and just one electron in the 4s because this gives it more stability. 8 a) [Ar]3d2 8 b) [Ar]3d7 8 c) [Ar]3d9 8 d) [Ar]3d8 • Neutral molecule or ion having a lone electron pair that can be used to form a bond to a metal ion. Monodentate ligand – one bond to a metal ion Bidentate ligand (chelate) – two bonds to a metal ion Polydentate ligand – more than two bonds to a metal ion • Transition metals can form complexes because their ions have a high charge density: o they have quite a large nuclear charge but are relatively small; o the 3d electrons are not so effective (as 2s or 2p electrons) at shielding the effect of the ionic charge which really comes from the nucleus. • This allows the transition metal ions to have a great polarising power and they can attract lone pairs from other atoms to form complexes. • Ions formed by a metal ion to which a number of ligands (molecules and/or negative ions) are bonded using a dative bond • Example: [Fe(H2O)6]3+ • Coordination number is the number of ligands that surround the metal ion [Fe(CN)6]3- [CuCl4]2- [Cu(NH3)4]2+ [Ag(NH3)2]+ Charge on the ion is the sum of all charges. Work out the charge of each metal ion in the complexes above. H2O OHNH3 ClBrCN- - - ligand aqua hydroxo ammine chloro bromo cyano number 1 - mono 2 - di 3 - tri 4 - tetra 5 - pent 6 – hex You name the ligand and its number first before naming the metal. You use the name of the metal if the ion is positive but use –ate ending if negative e.g. ferrate, cuprate, vanadate. Depends on coordination number • If 6 • If 4 • If 2 then shape = octahedral then shape = tetrahedral (or square planar = less common) then shape = linear • nuclear charge • oxidation state of ion (i.e. number of electrons) • ligand • coordination number • shape of the complex