Lecture 5
... comes from a 4S3/2-2D5/2 transition and has a critical density of 1.5 x 103 cm-3. The 6731Å line comes from the 4S3/2-2D3/2 transition with critical density 3.9 x 103 cm-3. Note that the line ratio is sensitivite to density between about 100 to 10,000 cm-3. ...
... comes from a 4S3/2-2D5/2 transition and has a critical density of 1.5 x 103 cm-3. The 6731Å line comes from the 4S3/2-2D3/2 transition with critical density 3.9 x 103 cm-3. Note that the line ratio is sensitivite to density between about 100 to 10,000 cm-3. ...
Introduction to Nanoscience
... could make a Cadillac that weighed fifty kilograms, or a full-sized sofa you could pick up with one hand. We could make surgical instruments of such precision and deftness that they could operate on the cells and even molecules from which we are made -- something well beyond today's medical technolo ...
... could make a Cadillac that weighed fifty kilograms, or a full-sized sofa you could pick up with one hand. We could make surgical instruments of such precision and deftness that they could operate on the cells and even molecules from which we are made -- something well beyond today's medical technolo ...
Chapter 3
... 76. What is the theoretical yield of chromium that can be produced by the reaction of 40.0 g of Cr2O3 with 8.00 g of aluminum according to the chemical equation below? 2Al + Cr2O3 Al2O3 + 2Cr A. 7.7 g B. 15.4 g C. 27.3 g D. 30.8 g E. 49.9 g 77. Hydrogen fluoride is used in the manufacture of Freo ...
... 76. What is the theoretical yield of chromium that can be produced by the reaction of 40.0 g of Cr2O3 with 8.00 g of aluminum according to the chemical equation below? 2Al + Cr2O3 Al2O3 + 2Cr A. 7.7 g B. 15.4 g C. 27.3 g D. 30.8 g E. 49.9 g 77. Hydrogen fluoride is used in the manufacture of Freo ...
HighFour Chemistry Round 1 Category C: Grades 9 – 10 Thursday
... In conclusion, iodine gas (I2) has the highest boiling point. This conclusion can be supported by a table where their actual boiling points are provided. ...
... In conclusion, iodine gas (I2) has the highest boiling point. This conclusion can be supported by a table where their actual boiling points are provided. ...
∙ ∙B x
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
∙ ∙B x
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
... The distribution of electrons in a molecule is never perfectly symmetrical – electrons move randomly within a molecule. It may happen that on one side of the molecule there is a higher electron density (This side is then slightly ..........................) and on the other side there is a ......... ...
Figure 4 - University of Wisconsin–Madison
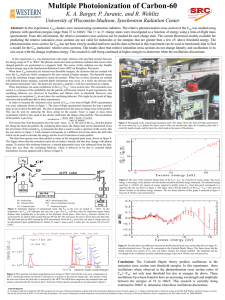
... photons with specified energies range from 37 to 160eV. The 1+ to 3+ charge states were investigated as a function of energy using a time-of-flight mass spectrometer. From this information, the relative ionization cross sections can be studied for each charge state. The current theoretical models av ...
... photons with specified energies range from 37 to 160eV. The 1+ to 3+ charge states were investigated as a function of energy using a time-of-flight mass spectrometer. From this information, the relative ionization cross sections can be studied for each charge state. The current theoretical models av ...
Unit chemical bonds
... • Which Has the largest Mass? 3 Mol of Magnesium or 1 mol of sucrose or 10 mol of helium ...
... • Which Has the largest Mass? 3 Mol of Magnesium or 1 mol of sucrose or 10 mol of helium ...
Question 1. Phosgene was used during the World War - IQ
... (b) Azobenzene is known to have greater delocalization of its π electrons than the hydrazobenzene. Provide an explanation for this statement based on non-hybridized atomic orbitals and N-N-C bond angles in each of these molecules. Question 9. The molecule of ethyl butanoate (C6H12O2) is responsible ...
... (b) Azobenzene is known to have greater delocalization of its π electrons than the hydrazobenzene. Provide an explanation for this statement based on non-hybridized atomic orbitals and N-N-C bond angles in each of these molecules. Question 9. The molecule of ethyl butanoate (C6H12O2) is responsible ...
02_Lecture_Presentation
... • Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms • These interactions usually result in atoms staying close together, held by attractions called chemical bonds ...
... • Atoms with incomplete valence shells can share or transfer valence electrons with certain other atoms • These interactions usually result in atoms staying close together, held by attractions called chemical bonds ...
isuintroduction
... temperature is.(2) For instance, 1 mol (which is the abbreviation of the mole) of a gas at 3000 K (K is the abbreviation of the Kelvin temperature scale) contains 6.02 x 1023 particles, while 1 mol of the same element in a solid state, at 273 K, would still be 6.02 x 1023 particles. Despite the atom ...
... temperature is.(2) For instance, 1 mol (which is the abbreviation of the mole) of a gas at 3000 K (K is the abbreviation of the Kelvin temperature scale) contains 6.02 x 1023 particles, while 1 mol of the same element in a solid state, at 273 K, would still be 6.02 x 1023 particles. Despite the atom ...
Chapter 10 The Periodic Law
... Polar covalent compounds are those in which the shared electron pairs are closer to one atom than to the other, making one part of the molecule relatively negative and another part relatively positive. ...
... Polar covalent compounds are those in which the shared electron pairs are closer to one atom than to the other, making one part of the molecule relatively negative and another part relatively positive. ...
Chapter 3 Crystallography and Diffraction Techniques
... Powder Diffraction File (International Center for Diffraction Data, USA), previously known as the ASTM or JCPDS file is an invaluable reference source for the identification of unknown crystalline materials. The file ...
... Powder Diffraction File (International Center for Diffraction Data, USA), previously known as the ASTM or JCPDS file is an invaluable reference source for the identification of unknown crystalline materials. The file ...
An element`s properties depend on the structure of its atoms
... • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of potential energy is called its energy level, or electron shell* ...
... • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of potential energy is called its energy level, or electron shell* ...
Adv review key
... _Group/Family 15_7. Name of the chemical family containing Nitrogen _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen Ionic bonding A) Electrons are transferred between atoms B) Valence electrons- ...
... _Group/Family 15_7. Name of the chemical family containing Nitrogen _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen Ionic bonding A) Electrons are transferred between atoms B) Valence electrons- ...
APS 1st semester exam review 2016
... _Group/Family 15_7. Name of the chemical family containing Nitrogen _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen Ionic bonding A) Electrons are transferred between atoms B) Valence electrons- ...
... _Group/Family 15_7. Name of the chemical family containing Nitrogen _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen Ionic bonding A) Electrons are transferred between atoms B) Valence electrons- ...
1 st Nine Weeks Study Guide for Chemistry
... E. Describe Rutherford’s Experiment and his discovery. Discovered nucleus-gold foil experiment, most of atom is empty space and discovered heavy mass in center of atom (nucleus) F. Describe JJ Thomson’s Experiment and his discovery. Discovered electrons , plum pudding model, used cathode ray tube. ...
... E. Describe Rutherford’s Experiment and his discovery. Discovered nucleus-gold foil experiment, most of atom is empty space and discovered heavy mass in center of atom (nucleus) F. Describe JJ Thomson’s Experiment and his discovery. Discovered electrons , plum pudding model, used cathode ray tube. ...
slides introducing IR/Raman of proteins
... Spectral Regions and Transitions • Infrared radiation excites molecular vibrations, i.e. stretching of bonds and deformation of bond angles. Molecule has 3N-6 internal degrees of freedom, N atoms. States characterize the bound ground state. • Radiation in the visible (Vis) and ultraviolet (UV) regi ...
... Spectral Regions and Transitions • Infrared radiation excites molecular vibrations, i.e. stretching of bonds and deformation of bond angles. Molecule has 3N-6 internal degrees of freedom, N atoms. States characterize the bound ground state. • Radiation in the visible (Vis) and ultraviolet (UV) regi ...
Metastable inner-shell molecular state

Metastable Innershell Molecular State (MIMS) is a class of ultra-high-energy short-lived molecules have the binding energy up to 1,000 times larger and bond length up to 100 times smaller than typical molecules. MIMS is formed by inner-shell electrons that are normally resistant to molecular formation. However, in stellar conditions, the inner-shell electrons become reactive to form molecular structures (MIMS) from combinations of all elements in the periodic table. MIMS upon dissociation can emit x-ray photons with energies up to 100 keV at extremely high conversion efficiencies from compression energy to photon energy. MIMS is predicted to exist and dominate radiation processes in extreme astrophysical environments, such as large planet cores, star interiors, and black hole and neutron star surroundings. There, MIMS is predicted to enable highly energy-efficient transformation of the stellar compression energy into the radiation energy.The right schematic illustration shows the proposed four stages of the K-shell MIMS (K-MIMS) formation and x-ray generation process. Stage I: Individual atoms are subjected to the stellar compression and ready for absorbing the compression energy. Stage II: The outer electron shells fuse together under increasing ""stellar"" pressure. Stage III: At the peak pressure, via pressure ionization K-shell orbits form the K-MIMS, which is vibrationally hot and encapsulated by a Rydberg-like pseudo-L-Shell structure. Stage IV: The K-MIMS cools down by ionizing (""boiling-off"") a number of pseudo-L-shell electrons and subsequent optical decay by emitting an x-ray photon. The dissociated atoms return their original atoms states and are ready for absorbing the compression energy.MIMS also can be readily produced in laboratory and industrial environments, such as hypervelocity particle impact, laser fusion and z-machine. MIMS can be exploited for highly energy-efficient production of high intensity x-ray beams for a wide range of innovative applications, such as photolithography, x-ray lasers, and inertial fusion.