- Bright Star Schools
... 2. Describe what an atom looks like in your own words (use the vocabulary) ...
... 2. Describe what an atom looks like in your own words (use the vocabulary) ...
Artificial Radioactivity Artificial Radioactivity And Q
... and so cause ionization, which is completed within about 10-15 s. Alternatively, the atomic electron may be excited to a higher state. These inelastic collisions are the most important mechanisms of energy loss. ...
... and so cause ionization, which is completed within about 10-15 s. Alternatively, the atomic electron may be excited to a higher state. These inelastic collisions are the most important mechanisms of energy loss. ...
pptx - Yale University
... In the last 25 years various manifestations of Scanning Probe Microscopy, such as AFM, STM, and SNOM, have enabled chemists to “feel” individual molecules and atoms. SPM techniques are not quite good enough yet to study how electrons are distributed in bonds. Because light is scattered predominantly ...
... In the last 25 years various manifestations of Scanning Probe Microscopy, such as AFM, STM, and SNOM, have enabled chemists to “feel” individual molecules and atoms. SPM techniques are not quite good enough yet to study how electrons are distributed in bonds. Because light is scattered predominantly ...
Chem Ch 4 test review
... 5. Define isotope, atomic number and mass number. 6. Write an isotope symbol. Which part is the atomic number (# of protons)? Which part is the mass number (# protons and neutrons)? 7. Compute the average atomic mass for an element computed, 8. Identify the 9 major areas of periodic table and select ...
... 5. Define isotope, atomic number and mass number. 6. Write an isotope symbol. Which part is the atomic number (# of protons)? Which part is the mass number (# protons and neutrons)? 7. Compute the average atomic mass for an element computed, 8. Identify the 9 major areas of periodic table and select ...
Topic Book periodicity
... Oxide: Metal oxides are ionic (explains conductivity, m.p. and b.p.) and react with water to form alkaline solutions. SiO2 is covalent macromolecular, and the remaining oxides are simple covalent. Oxides of period 3 elements are solid from Na2O to P4O10 and P4O6, while SO3 and C. Al2O3 and SiO2 doC ...
... Oxide: Metal oxides are ionic (explains conductivity, m.p. and b.p.) and react with water to form alkaline solutions. SiO2 is covalent macromolecular, and the remaining oxides are simple covalent. Oxides of period 3 elements are solid from Na2O to P4O10 and P4O6, while SO3 and C. Al2O3 and SiO2 doC ...
Ch 8 AP Practice
... (A) CO2 (B) H2O (C) CH4 (D) C2H4 (E) PH3 3. The molecule with only one double bond 4. The molecule with the largest dipole moment 5. The molecule that has trigonal pyramidal geometry 53. According to the VSEPR model, the progressive decrease in the bond angles in the series of molecules CH4, NH3, an ...
... (A) CO2 (B) H2O (C) CH4 (D) C2H4 (E) PH3 3. The molecule with only one double bond 4. The molecule with the largest dipole moment 5. The molecule that has trigonal pyramidal geometry 53. According to the VSEPR model, the progressive decrease in the bond angles in the series of molecules CH4, NH3, an ...
Lecture 1.1 Some preliminary chemistry knowledge, ppt file
... atom, the single electron is held in its orbital by its attraction to the proton in the nucleus. ...
... atom, the single electron is held in its orbital by its attraction to the proton in the nucleus. ...
Glossary
... Bond order − a quantitative measure of bond strength defined as one-half the difference between the number of electrons in bonding orbitals and the number in antibonding orbitals. ...
... Bond order − a quantitative measure of bond strength defined as one-half the difference between the number of electrons in bonding orbitals and the number in antibonding orbitals. ...
Electrons_Holes
... • All electrons with the same energy and momentum are considered to be equal. • All holes with the same energy and momentum are considered to be equal. ▫ However, this is not true in magnetic semiconductors, such as MnGaAs, where spin up electrons are different from spin down electrons and similarly ...
... • All electrons with the same energy and momentum are considered to be equal. • All holes with the same energy and momentum are considered to be equal. ▫ However, this is not true in magnetic semiconductors, such as MnGaAs, where spin up electrons are different from spin down electrons and similarly ...
Chemistry Final Study Guide
... 39. An __________ forms when an atom gains or loses electrons. 40. Elements are organized by atomic number on the __________ __________. 41. The vertical columns are called __________, and elements within each of these have similar properties. 42. The horizontal rows are called __________, and each ...
... 39. An __________ forms when an atom gains or loses electrons. 40. Elements are organized by atomic number on the __________ __________. 41. The vertical columns are called __________, and elements within each of these have similar properties. 42. The horizontal rows are called __________, and each ...
3. atomic structure
... In this class we will be performing an experiment called the “Flame Test”. We will be heating up metal powders in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific ...
... In this class we will be performing an experiment called the “Flame Test”. We will be heating up metal powders in order to excite the electrons to jump from a lower energy level to a higher energy level. When an electron returns from a higher energy state to a lower energy state, it emits a specific ...
history of the atom ppt student copy
... -modified CRT with poles (magnetic field) to attract cathode rays. - passed electricity through a gas at first; then used several samples of other elements. -behavior was same for all elements - rays were attracted to the anode (+). (__________________________) - Concluded that _____________________ ...
... -modified CRT with poles (magnetic field) to attract cathode rays. - passed electricity through a gas at first; then used several samples of other elements. -behavior was same for all elements - rays were attracted to the anode (+). (__________________________) - Concluded that _____________________ ...
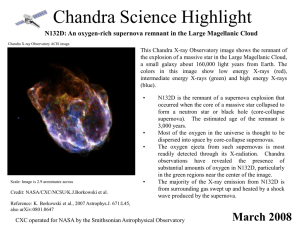
PowerPoint - Chandra X
... occurred when the core of a massive star collapsed to form a neutron star or black hole (core-collapse supernova). The estimated age of the remnant is 3,000 years. Most of the oxygen in the universe is thought to be dispersed into space by core-collapse supernovas. The oxygen ejecta from such supern ...
... occurred when the core of a massive star collapsed to form a neutron star or black hole (core-collapse supernova). The estimated age of the remnant is 3,000 years. Most of the oxygen in the universe is thought to be dispersed into space by core-collapse supernovas. The oxygen ejecta from such supern ...
AP Chemistry
... maximum opportunity for students to learn a variety of skills and those facts, principles, and concepts of chemistry covered in lectures, reading, and discussion. In addition, the laboratory exercises are used to present novel material not covered in other parts of the course. Students develop the s ...
... maximum opportunity for students to learn a variety of skills and those facts, principles, and concepts of chemistry covered in lectures, reading, and discussion. In addition, the laboratory exercises are used to present novel material not covered in other parts of the course. Students develop the s ...
Draw atomic models showing the appropriate number of electrons
... 1. How strongly an atom is able to tug on bonding electrons ...
... 1. How strongly an atom is able to tug on bonding electrons ...
4 - College of Arts and Sciences
... • Atomic Theory of Matter by Dalton • Atomic Structure by Rutherford – atomic numbers, mass numbers, and isotopes – notation ...
... • Atomic Theory of Matter by Dalton • Atomic Structure by Rutherford – atomic numbers, mass numbers, and isotopes – notation ...
Chapter 12: Basic Review Worksheet
... attaining an octet of electrons important for an atom when it forms bonds to other atoms? 12. What is a bonding pair of electrons? What is nonbonding (or lone) pair of electrons? ...
... attaining an octet of electrons important for an atom when it forms bonds to other atoms? 12. What is a bonding pair of electrons? What is nonbonding (or lone) pair of electrons? ...
SAXS on lipid structures
... for the adjusted angles of incidence, photons with different energies do not experience constructive interference and thus are absorbed by or passing through the mirror. Due to the parabolic alignment of the layers, all incoming radiation is reflected into the same direction and the beam gets collim ...
... for the adjusted angles of incidence, photons with different energies do not experience constructive interference and thus are absorbed by or passing through the mirror. Due to the parabolic alignment of the layers, all incoming radiation is reflected into the same direction and the beam gets collim ...
SCH3U Course Review
... increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
... increase with increasing atomic radii decrease with increasing nuclear charge decrease across a period from left to right increase across a period from left to right increase as you go down a family ...
bonding notes for votech
... Na +1 and F -1 combine to form NaF (1:1 ratio) Na will lose 1e- and F will gain 1eOverall charge on NaF is (+1 + -1 = 0) Swap Charges: Na +1 ...
... Na +1 and F -1 combine to form NaF (1:1 ratio) Na will lose 1e- and F will gain 1eOverall charge on NaF is (+1 + -1 = 0) Swap Charges: Na +1 ...
Metastable inner-shell molecular state

Metastable Innershell Molecular State (MIMS) is a class of ultra-high-energy short-lived molecules have the binding energy up to 1,000 times larger and bond length up to 100 times smaller than typical molecules. MIMS is formed by inner-shell electrons that are normally resistant to molecular formation. However, in stellar conditions, the inner-shell electrons become reactive to form molecular structures (MIMS) from combinations of all elements in the periodic table. MIMS upon dissociation can emit x-ray photons with energies up to 100 keV at extremely high conversion efficiencies from compression energy to photon energy. MIMS is predicted to exist and dominate radiation processes in extreme astrophysical environments, such as large planet cores, star interiors, and black hole and neutron star surroundings. There, MIMS is predicted to enable highly energy-efficient transformation of the stellar compression energy into the radiation energy.The right schematic illustration shows the proposed four stages of the K-shell MIMS (K-MIMS) formation and x-ray generation process. Stage I: Individual atoms are subjected to the stellar compression and ready for absorbing the compression energy. Stage II: The outer electron shells fuse together under increasing ""stellar"" pressure. Stage III: At the peak pressure, via pressure ionization K-shell orbits form the K-MIMS, which is vibrationally hot and encapsulated by a Rydberg-like pseudo-L-Shell structure. Stage IV: The K-MIMS cools down by ionizing (""boiling-off"") a number of pseudo-L-shell electrons and subsequent optical decay by emitting an x-ray photon. The dissociated atoms return their original atoms states and are ready for absorbing the compression energy.MIMS also can be readily produced in laboratory and industrial environments, such as hypervelocity particle impact, laser fusion and z-machine. MIMS can be exploited for highly energy-efficient production of high intensity x-ray beams for a wide range of innovative applications, such as photolithography, x-ray lasers, and inertial fusion.