Chemical Formulas and Equations
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
... chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence. ...
Prior knowledge catch-up student sheet for Chapter 3 Quantitative
... For example, the atomic number of sodium is 11 and the mass number is 23. Number of protons = 11 Number of electrons = 11 Number of neutrons = 23 − 11 = 12 Chemical reactions can be represented using a formula to show reactants and products in a chemical equation, with an arrow in between. An equati ...
... For example, the atomic number of sodium is 11 and the mass number is 23. Number of protons = 11 Number of electrons = 11 Number of neutrons = 23 − 11 = 12 Chemical reactions can be represented using a formula to show reactants and products in a chemical equation, with an arrow in between. An equati ...
Bonding and Structure Organic Molecular Structure
... • Rotation around single C-C bonds does NOT generate a new chemical structure, just the same structure drawn a different way (later we will see that these different ways of drawing the structures are called conformers) ...
... • Rotation around single C-C bonds does NOT generate a new chemical structure, just the same structure drawn a different way (later we will see that these different ways of drawing the structures are called conformers) ...
Experimental and Theoretical Charge Density Analysis of a
... electron distribution, a precise charge density analysis (either experimental or theoretical) is a method of choice to recover molecular properties. In particular, it is of interest to know how the two CH2 carbon units present in compound BEST between the bromine and the sulfur atom are different fro ...
... electron distribution, a precise charge density analysis (either experimental or theoretical) is a method of choice to recover molecular properties. In particular, it is of interest to know how the two CH2 carbon units present in compound BEST between the bromine and the sulfur atom are different fro ...
g - Porterville College Home
... that oxygen is paired with. Larger subscript of oxygen in a series name ends in “–ate.” Smaller subscript of oxygen in a series name ends in “–ite.” See Oxyanion tips. 2. Other: Some other polyatomic anions include CNcyanide, OH- hydroxide, peroxide O222. Acid: Last word of acid name is always “acid ...
... that oxygen is paired with. Larger subscript of oxygen in a series name ends in “–ate.” Smaller subscript of oxygen in a series name ends in “–ite.” See Oxyanion tips. 2. Other: Some other polyatomic anions include CNcyanide, OH- hydroxide, peroxide O222. Acid: Last word of acid name is always “acid ...
CHEM3023: Spins, Atoms and Molecules
... • Is a fundamental law of nature: It can not be proved, but we know it works. Newton's second law of motion (F=m a) is another example of a law of nature. • Applies at the microscopic scale: electrons, atoms, molecules, etc. • What information can it provide? Every property that can be measured expe ...
... • Is a fundamental law of nature: It can not be proved, but we know it works. Newton's second law of motion (F=m a) is another example of a law of nature. • Applies at the microscopic scale: electrons, atoms, molecules, etc. • What information can it provide? Every property that can be measured expe ...
CHAPTER 2
... bonding where an electron _____________________ reacts with another ________________. A COVALENT BOND is the result of the _____________________ of one or more electron _______________ between two ______________________ atoms. When molecular fluorine (F2) is formed, each atom _____________ an elec ...
... bonding where an electron _____________________ reacts with another ________________. A COVALENT BOND is the result of the _____________________ of one or more electron _______________ between two ______________________ atoms. When molecular fluorine (F2) is formed, each atom _____________ an elec ...
Chemistry (Theory)
... (b) (i) Nitrogen is chemically less reactive. This is because of the high stability of its molecule, N2. In N2, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form p ...
... (b) (i) Nitrogen is chemically less reactive. This is because of the high stability of its molecule, N2. In N2, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form p ...
Combining and Choosing Analytical Techniques
... This causes the ions to move in a curved path with a radius that depends upon the m/z ratio of the ions. Only ions moving in a curved path of a particular radius corresponding to a fixed m/z ratio will reach the collector The collector measures the current due to the ions reaching the detector and t ...
... This causes the ions to move in a curved path with a radius that depends upon the m/z ratio of the ions. Only ions moving in a curved path of a particular radius corresponding to a fixed m/z ratio will reach the collector The collector measures the current due to the ions reaching the detector and t ...
CHAPTER 5
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
Final Exam 2004
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
Topic 4: Classifying Elements What did the early chemists use to
... We usually refer to compounds containing HYDROGEN by their COMMON name. All compounds containing hydrogen are MOLECULAR compounds. How do we indicate the physical state of a compound? (something is writte ...
... We usually refer to compounds containing HYDROGEN by their COMMON name. All compounds containing hydrogen are MOLECULAR compounds. How do we indicate the physical state of a compound? (something is writte ...
4b. Orbital Diagrams
... Orbital Diagrams • Use individual orbitals • Give subshell arrangement • Each orbital takes one electron before any other orbital in the same subshell can receive a second electron ...
... Orbital Diagrams • Use individual orbitals • Give subshell arrangement • Each orbital takes one electron before any other orbital in the same subshell can receive a second electron ...
chapter_10au use in class
... Polar Covalent Bonds Most chemical bonds fall between 100% ionic and 100% covalent. In a polar covalent bond, electrons are not shared equally between two atom. In such a bond, electrons are displaced toward the more nonmetallic element. ...
... Polar Covalent Bonds Most chemical bonds fall between 100% ionic and 100% covalent. In a polar covalent bond, electrons are not shared equally between two atom. In such a bond, electrons are displaced toward the more nonmetallic element. ...
Lecture 5
... 3. You are familiar with the crystal structures of some simple crystals, such as those we recently discussed. Note: If you are weak on this or need a review, please get & read an undergraduate solid state book! 4. In a solid, the electronic energy levels form into regions of allowed energy (bands) & ...
... 3. You are familiar with the crystal structures of some simple crystals, such as those we recently discussed. Note: If you are weak on this or need a review, please get & read an undergraduate solid state book! 4. In a solid, the electronic energy levels form into regions of allowed energy (bands) & ...
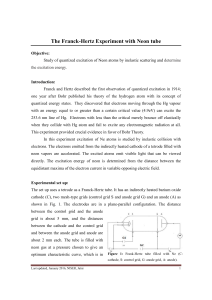
The Franck-Hertz Experiment with Neon tube
... Franck and Hertz described the first observation of quantized excitation in 1914; ...
... Franck and Hertz described the first observation of quantized excitation in 1914; ...
ppt
... Most of the mass of an atom is in the small, dense nucleus. ● The radius of an atom is about 100,000 times larger than the radius of the nucleus. ● Electrons are located around the nucleus in orbitals. ● Orbitals – not distinct like planetary orbits, but 3-D regions where electrons can probably be ...
... Most of the mass of an atom is in the small, dense nucleus. ● The radius of an atom is about 100,000 times larger than the radius of the nucleus. ● Electrons are located around the nucleus in orbitals. ● Orbitals – not distinct like planetary orbits, but 3-D regions where electrons can probably be ...
Quantum Numbers “Where are the Electrons?”
... Quantum numbers are used to describe atomic orbitals and the electrons in them. There are 4 quantum numbers: o The principal quantum number (n), indicates the main energy level occupied by the electron. n = a whole number such as 1, 2, 3, 4 n tells the distance from the nucleus and the energy of ...
... Quantum numbers are used to describe atomic orbitals and the electrons in them. There are 4 quantum numbers: o The principal quantum number (n), indicates the main energy level occupied by the electron. n = a whole number such as 1, 2, 3, 4 n tells the distance from the nucleus and the energy of ...
Laboratory 1
... 1. Plot the window function for the phonon transport 2 at different temperatures. k T ...
... 1. Plot the window function for the phonon transport 2 at different temperatures. k T ...
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction. The strength of chemical bonds varies considerably; there are ""strong bonds"" such as covalent or ionic bonds and ""weak bonds"" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemists to predict the strength, directionality, and polarity of bonds. The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization and resonance, and the linear combination of atomic orbitals molecular orbital method which includes ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.