Examples
... You need to find the lowest number of each ion to make it neutral for example: Na+ and O2 2 sodium for every one oxygen Na2O ...
... You need to find the lowest number of each ion to make it neutral for example: Na+ and O2 2 sodium for every one oxygen Na2O ...
Batteries are all over the place -- in our cars, our
... negative and positive terminals, the electrons will flow from the negative to the positive terminal as fast as they can (and wear out the battery very quickly -- this also tends to be dangerous, especially with large batteries, so it is not something you want to be doing). Normally, you connect some ...
... negative and positive terminals, the electrons will flow from the negative to the positive terminal as fast as they can (and wear out the battery very quickly -- this also tends to be dangerous, especially with large batteries, so it is not something you want to be doing). Normally, you connect some ...
Topic 5 Energetics File
... 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stable than reactants. Gibb’s free energy: Must be negative for reaction to be spontaneous. ΔG = ΔH – TΔS Hess’ law: Enthalpy change for a reaction depends only on difference between enthalpy of prod ...
... 3) increase of temperature Exothermic: A reaction in which energy is evolved. ΔH is –. Products more stable than reactants. Gibb’s free energy: Must be negative for reaction to be spontaneous. ΔG = ΔH – TΔS Hess’ law: Enthalpy change for a reaction depends only on difference between enthalpy of prod ...
VCAA Study Design - Chemistry Education Association
... • lack of awareness of the differences between discharging and recharging in terms of the direction of electron flow, and that electrons always move from the site of oxidation (anode) to the site of reduction (cathode) • inability to correctly explain the changes in the rates of the forward and reve ...
... • lack of awareness of the differences between discharging and recharging in terms of the direction of electron flow, and that electrons always move from the site of oxidation (anode) to the site of reduction (cathode) • inability to correctly explain the changes in the rates of the forward and reve ...
Reactions and Stoichiometry Practice Problems
... 25) How many grams of NO are required to produce 145 g of N2 in the following unbalanced reaction? NH3 + ...
... 25) How many grams of NO are required to produce 145 g of N2 in the following unbalanced reaction? NH3 + ...
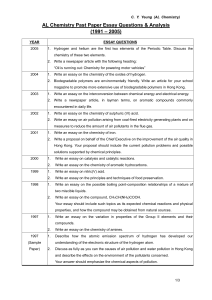
AL Chemistry Past paper essay questions
... Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
... Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
Chemistry 8.2
... burning charcoal are the products of a combustion reaction. Combustion is one of the five general types of chemical reactions. If you can recognize a reaction as being a particular type, you may be able to predict the products of the reaction. ...
... burning charcoal are the products of a combustion reaction. Combustion is one of the five general types of chemical reactions. If you can recognize a reaction as being a particular type, you may be able to predict the products of the reaction. ...
Solids Chemistry XII - The Gurukul Institute
... Which concentration term remain unaffected for a solution when its temperature changes? Suppose a solid solution is formed between two substances, one of whose particles are very large and the other whose particles are very small, what kind of solid solution in this likely to be? Define ‘mole fract ...
... Which concentration term remain unaffected for a solution when its temperature changes? Suppose a solid solution is formed between two substances, one of whose particles are very large and the other whose particles are very small, what kind of solid solution in this likely to be? Define ‘mole fract ...
111 Exam I Outline
... IV. LIMITING REACTANTS When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the re ...
... IV. LIMITING REACTANTS When most reactions are performed, some of the reactants is usually present in excess of the amount needed. If the reaction goes to completion, then some of this excess reactant will be left-over. The limiting reactant is the reactant used-up completely and it "limits" the re ...
Section 2 Types of Chemical Reactions
... Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to give a balanced ...
... Write word and formula equations for the chemical reaction that occurs when solid sodium oxide is added to water at room temperature and forms sodium hydroxide (dissolved in the water). Include symbols for physical states in the formula equation. Then balance the formula equation to give a balanced ...
GCE Getting Started - Edexcel
... models showing the shapes of a variety of molecules. They then draw a dot-and-cross diagram for each molecule and produce a summary that links number of electron pairs to shape. Selfassess against VSEPR rules. ...
... models showing the shapes of a variety of molecules. They then draw a dot-and-cross diagram for each molecule and produce a summary that links number of electron pairs to shape. Selfassess against VSEPR rules. ...
MERIDIAN PUBLIC SCHOOL DISTRICT
... Atomic number, atomic mass, mass number, and number of protons, electrons, and neutrons in isotopes of elements Chemical characteristics of each region Periodic properties (e.g., metal/nonmetal/metalloid behavior, electrical/heat conductivity, electronegativity, electron affinity, ionization energy, ...
... Atomic number, atomic mass, mass number, and number of protons, electrons, and neutrons in isotopes of elements Chemical characteristics of each region Periodic properties (e.g., metal/nonmetal/metalloid behavior, electrical/heat conductivity, electronegativity, electron affinity, ionization energy, ...
Lipid Hydroperoxide Activation of N-Hydroxy-N
... INTRODUCTION The exact chemical events that result in the activation of the arylamine carcinogens to the ultimate reactive chemical form are still an open question. However, much has been learned in recent years (see Refs. 15, 16, and 21 for re views). For example, it was discovered in 1960 (5) that ...
... INTRODUCTION The exact chemical events that result in the activation of the arylamine carcinogens to the ultimate reactive chemical form are still an open question. However, much has been learned in recent years (see Refs. 15, 16, and 21 for re views). For example, it was discovered in 1960 (5) that ...
THERMOCHEMISTRY
... 1. Two solutions, 100.0 mL of 1.00 M AgNO3 and 100.0 mL of 1.00 M NaCl, both initially at 22.4 °C, are added to a calorimeter and allowed to react. The temperature rises to 30.2 °C. Calculate the heat of reaction in kJ/mol AgCl produced. (Assume density and specific heat of solution to be the ...
... 1. Two solutions, 100.0 mL of 1.00 M AgNO3 and 100.0 mL of 1.00 M NaCl, both initially at 22.4 °C, are added to a calorimeter and allowed to react. The temperature rises to 30.2 °C. Calculate the heat of reaction in kJ/mol AgCl produced. (Assume density and specific heat of solution to be the ...
A Classification of AP Chemistry Reactions
... The second type of redox that involve oxygen-containing compounds such as nitrates, sulfates, permanganates, dichromates, etc. First of all, since these are redox reactions, one thing must be oxidized and another must be reduced. Jotting down oxidation numbers can be helpful. Second, almost all of t ...
... The second type of redox that involve oxygen-containing compounds such as nitrates, sulfates, permanganates, dichromates, etc. First of all, since these are redox reactions, one thing must be oxidized and another must be reduced. Jotting down oxidation numbers can be helpful. Second, almost all of t ...
The Designer-Synthesizer Debate: What Does a
... Medicinal Chemistry Design & Synthesis | March 2016 | © 2016 AbbVie ...
... Medicinal Chemistry Design & Synthesis | March 2016 | © 2016 AbbVie ...
Practice Test: Equilibrium
... 6. If the system is at equilibrium and 5.0 moles of chlorine are added, what will be the new Keq? ______ 7. If the system is at equilibrium and the container is doubled in size, what will be the new Keq? ______ 8. What will happen to Keq if the temperature is lowered? ____________ 9. In summary, wha ...
... 6. If the system is at equilibrium and 5.0 moles of chlorine are added, what will be the new Keq? ______ 7. If the system is at equilibrium and the container is doubled in size, what will be the new Keq? ______ 8. What will happen to Keq if the temperature is lowered? ____________ 9. In summary, wha ...
Chemistry 201 - Department of Chemistry | Oregon State University
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
chapter 21 chemistry of the main-group elements i
... (M) When BeCl2 4H 2 O is heated, it decomposes to Be(OH)2(s), H2O(g), and HCl(g), as discussed in Section 21-3. BeCl2 4H 2 O comprises [Be(H2O)4]2+ and Cl− ions. Because of the high polarizing power of Be2+, it is difficult to remove the coordinated water molecules by heating the solid and the a ...
... (M) When BeCl2 4H 2 O is heated, it decomposes to Be(OH)2(s), H2O(g), and HCl(g), as discussed in Section 21-3. BeCl2 4H 2 O comprises [Be(H2O)4]2+ and Cl− ions. Because of the high polarizing power of Be2+, it is difficult to remove the coordinated water molecules by heating the solid and the a ...