BSPH 111 - Refresher Chemistry
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
M.Sc. Part-I Chemistry - North Maharashtra University
... NORTH MAHARASHTRA UNIVERSITY JALGAON M. Sc. - Chemistry ( Part- I ) CH-P-1 Physical Chemistry Practical Skeleton for question paper of CH – P -1 ...
... NORTH MAHARASHTRA UNIVERSITY JALGAON M. Sc. - Chemistry ( Part- I ) CH-P-1 Physical Chemistry Practical Skeleton for question paper of CH – P -1 ...
Exam No. 1
... 26- A 25.00 mL sample of HCl solution requires 24.16 mL of 0.106 M sodium hydroxide (NaOH) for complete neutralization; HCl(aq) + NaOH(aq) → NaCl(aq) The concentration of the original HCl solution is: (a) 0.204 M **(c) 0.102 M ...
... 26- A 25.00 mL sample of HCl solution requires 24.16 mL of 0.106 M sodium hydroxide (NaOH) for complete neutralization; HCl(aq) + NaOH(aq) → NaCl(aq) The concentration of the original HCl solution is: (a) 0.204 M **(c) 0.102 M ...
Step 2
... call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded molecules have low melting and boiling points (i.e. they ...
... call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded molecules have low melting and boiling points (i.e. they ...
Step 2 - The Grange School Blogs
... call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded molecules have low melting and boiling points (i.e. they ...
... call it ____________ BONDING. This type of bonding normally occurs between _______ atoms. It causes the atoms in a molecule to be held together very strongly but there are ____ forces between individual molecules. This is why covalently-bonded molecules have low melting and boiling points (i.e. they ...
Chemical Equilibria - Beck-Shop
... Q: Why are the terms involving solids and liquids not included in the equilibrium constant expression for heterogeneous equilibrium? A: For a given temperature, the saturated vapour pressures of solids (and that of liquids) are constant. In addition, even though their actual amounts may change, both ...
... Q: Why are the terms involving solids and liquids not included in the equilibrium constant expression for heterogeneous equilibrium? A: For a given temperature, the saturated vapour pressures of solids (and that of liquids) are constant. In addition, even though their actual amounts may change, both ...
University of Lusaka
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
... with different numbers of neutrons are isotopes of that element. Isotopes typically exhibit similar chemical behaviour to each other. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. Electrons have such little mass that they exhibit properties ...
Spring 2005
... 18. While in lab, your lab partner accidentally slops 10 mL of 1 M HCl onto your bare arm. a. (3 pts) What action should you take? b. (3 pts) What action should your lab partner take? 19. (6 pts) Draw the Lewis structure for H2SO4 including any resonance structures. 20. (10 pts) Ammonia is produced ...
... 18. While in lab, your lab partner accidentally slops 10 mL of 1 M HCl onto your bare arm. a. (3 pts) What action should you take? b. (3 pts) What action should your lab partner take? 19. (6 pts) Draw the Lewis structure for H2SO4 including any resonance structures. 20. (10 pts) Ammonia is produced ...
fulltext
... contributes with a distinct side chain with properties elegantly chosen by evolution. The proteins fold to form ordered vital structures, biologically functional units. The structures formed by the amino acid residues are many; the most common motifs are the α-helix and the β-sheet which are usually ...
... contributes with a distinct side chain with properties elegantly chosen by evolution. The proteins fold to form ordered vital structures, biologically functional units. The structures formed by the amino acid residues are many; the most common motifs are the α-helix and the β-sheet which are usually ...
Chapter 7 - NordoniaHonorsChemistry
... Yes, food decomposing and combining Digestion of food with stomach acid Dissolving sugar in water No, molecules still same ...
... Yes, food decomposing and combining Digestion of food with stomach acid Dissolving sugar in water No, molecules still same ...
AS Specification pdf | AS/A level
... This specification provides a suitable foundation for the study of chemistry at A level. In addition, the specification provides a coherent, satisfying and worthwhile course of study for learners who do not progress to further study in this subject. This specification is not age specific and, as suc ...
... This specification provides a suitable foundation for the study of chemistry at A level. In addition, the specification provides a coherent, satisfying and worthwhile course of study for learners who do not progress to further study in this subject. This specification is not age specific and, as suc ...
Downloaded on 2017-02
... to two precursor gases, separated by purges, completes one ALD cycle. Despite remarkable achievements in characterising these sub-nanometre thin layers during exposure 6,7 and in situ to the reactor, 8 there is still little understanding of the crucial processes of saturation and re-activation. This ...
... to two precursor gases, separated by purges, completes one ALD cycle. Despite remarkable achievements in characterising these sub-nanometre thin layers during exposure 6,7 and in situ to the reactor, 8 there is still little understanding of the crucial processes of saturation and re-activation. This ...
Title
... Criterion Discipline: General Chemistry II On these test 2x8, i.e. 16 points can be gathered, which is counted into the final mark of Medical Chemistry. One should reach at least the 30% (5 points) on the two test altogether and should participate on the 75% of the classes. To fulfil the requiremen ...
... Criterion Discipline: General Chemistry II On these test 2x8, i.e. 16 points can be gathered, which is counted into the final mark of Medical Chemistry. One should reach at least the 30% (5 points) on the two test altogether and should participate on the 75% of the classes. To fulfil the requiremen ...
Chapter 17: Reaction Energy and Reaction Kinetics
... compound has a high negative heat of formation. Such compounds are very stable. Once they start, the reactions forming them usually proceed vigorously and without outside assistance. Elements in their standard states are defined as having ∆H 0f = 0. The ∆H 0f of carbon dioxide is −393.5 kJ/mol of ga ...
... compound has a high negative heat of formation. Such compounds are very stable. Once they start, the reactions forming them usually proceed vigorously and without outside assistance. Elements in their standard states are defined as having ∆H 0f = 0. The ∆H 0f of carbon dioxide is −393.5 kJ/mol of ga ...
ii. year course contents
... To teach the basic analytical chemistry concepts to the chemistry students and gain some skills with methods for the qualitative and quantitative analysis of samples. Chemistry students who will be work in research institution and industry, learn basic analytical chemistry concepts, errors in chemic ...
... To teach the basic analytical chemistry concepts to the chemistry students and gain some skills with methods for the qualitative and quantitative analysis of samples. Chemistry students who will be work in research institution and industry, learn basic analytical chemistry concepts, errors in chemic ...
Chapter 4
... electrons cancel. In this case, multiply the reduction by 3 and the oxidation by 4 for a total of 24 electrons on each side. 3 x [8 OH- + N2H4 → 2 NO + 8 e- + 6 H2O] i.e., 24 OH- + 3 N2H4 → 6 NO + 24 e- + 18 H2O 4 x [3 H2O + 6 e- + BrO3- → Br- + 6 OH-] i.e., 12 H2O + 24 e- + 4 BrO3- → 4 Br- + 24 ...
... electrons cancel. In this case, multiply the reduction by 3 and the oxidation by 4 for a total of 24 electrons on each side. 3 x [8 OH- + N2H4 → 2 NO + 8 e- + 6 H2O] i.e., 24 OH- + 3 N2H4 → 6 NO + 24 e- + 18 H2O 4 x [3 H2O + 6 e- + BrO3- → Br- + 6 OH-] i.e., 12 H2O + 24 e- + 4 BrO3- → 4 Br- + 24 ...
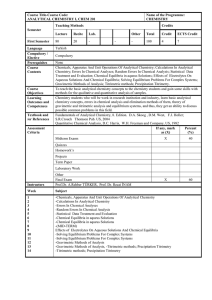
Chemistry – V – BSC – 503
... (III) olhard’s method with use of proper indicator, graph and it’s practical application Examples of calculation based on pH, Normality, Molarity,Ksp etc. ...
... (III) olhard’s method with use of proper indicator, graph and it’s practical application Examples of calculation based on pH, Normality, Molarity,Ksp etc. ...
CHEMICAL REACTIONS
... H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) to make a second H2O molecule. ...
... H2O. Then, the remaining oxygen atom combines with two more hydrogen atoms (from another H2 molecule) to make a second H2O molecule. ...