Unique Solutions
... Chemical reactions in which reactants gain hydrogen are reduction reactions. Similarly, the reaction in which reactants loose oxygen atom to form product is also ...
... Chemical reactions in which reactants gain hydrogen are reduction reactions. Similarly, the reaction in which reactants loose oxygen atom to form product is also ...
CHEMISTRY EXAM 2 REVIEW
... CHEMISTRY EXAM 2 REVIEW Name_____________________________ Per ____ Periodic Table, Physical and Chemical Properties, Changes, and Reactions Guardian Signature: _________________________________________________________________ My child completed this review and studied for at least 30 minutes. Define ...
... CHEMISTRY EXAM 2 REVIEW Name_____________________________ Per ____ Periodic Table, Physical and Chemical Properties, Changes, and Reactions Guardian Signature: _________________________________________________________________ My child completed this review and studied for at least 30 minutes. Define ...
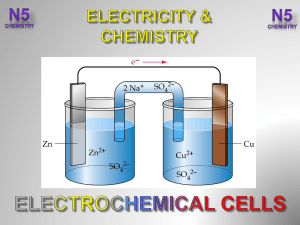
3.-Electrochemical-Cells-V2-
... After completing this topic you should be able to : • State electricity can be produced in a cell by connecting two different metals in solutions of their metal ions. ...
... After completing this topic you should be able to : • State electricity can be produced in a cell by connecting two different metals in solutions of their metal ions. ...
Chem vocab quiz definitons
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
Click here for the Reaction NOTES Handout
... products but at a slower pace and with more steps. Usually burns with blue flame. C10H8 + 12 O2 → 10 CO2 + 4 H2O (burning of naphthalene) b) Incomplete – not a lot of O2 , products will be CO and H2O unless otherwise specified, charcoal (Carbon) can be made by this process. Usually burns with yellow ...
... products but at a slower pace and with more steps. Usually burns with blue flame. C10H8 + 12 O2 → 10 CO2 + 4 H2O (burning of naphthalene) b) Incomplete – not a lot of O2 , products will be CO and H2O unless otherwise specified, charcoal (Carbon) can be made by this process. Usually burns with yellow ...
Chapter 2 Outline
... steroid based e.g. testosterone, progesterone C. Proteins – structural building blocks of the body 1. composed of amino acid monomers 2. Chemical composition CHON 3. Enzymes are protein catalysts a. Substrate – material enzyme is working on b. Binding site (active site) – place where substrate bonds ...
... steroid based e.g. testosterone, progesterone C. Proteins – structural building blocks of the body 1. composed of amino acid monomers 2. Chemical composition CHON 3. Enzymes are protein catalysts a. Substrate – material enzyme is working on b. Binding site (active site) – place where substrate bonds ...
chemical reaction
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and iodine. ...
... • 3. Describe the difference between single- and doubledisplacement reactions. • 4. Write the balanced equation in which potassium iodide, KI, reacts with chlorine to form potassium chloride, KCl, and iodine. ...
Packet 2- Chemistry of Life
... 1. Proteins are molecular machines and the way they are SHAPED determines what they can DO. A. Review: Protein structure i. Primary structure: String of amino acids ii. Secondary structure: The string is folded in some way (beta pleated sheets or alpha helices) iii. Tertiary structure: The fold ...
... 1. Proteins are molecular machines and the way they are SHAPED determines what they can DO. A. Review: Protein structure i. Primary structure: String of amino acids ii. Secondary structure: The string is folded in some way (beta pleated sheets or alpha helices) iii. Tertiary structure: The fold ...
Chapter 2 Chemical Reactions
... in water, an aqueous solution: NaCl(aq) is a salt water solution ...
... in water, an aqueous solution: NaCl(aq) is a salt water solution ...
Objective: The objective of the lab is to study the types of reactions
... A dissociation reaction is one that forms ions. If a molecule is made up on ions it might be able to be dissolved in water. Table salt (NaCl) is an example of a compound that is easily split into the Na+1 and the Cl-1 ions that make it up. Ions are charged particles that are very much similar to an ...
... A dissociation reaction is one that forms ions. If a molecule is made up on ions it might be able to be dissolved in water. Table salt (NaCl) is an example of a compound that is easily split into the Na+1 and the Cl-1 ions that make it up. Ions are charged particles that are very much similar to an ...
Lecture 5 – Chemical Reactions
... Chemical equations embody a fundamental law of nature called the law of conservation of matter. a. The law states, that in a chemical reaction atoms are neither created or destroyed, only rearranged. b. All of the matter present in the reactants is also present in the products of the reaction. ...
... Chemical equations embody a fundamental law of nature called the law of conservation of matter. a. The law states, that in a chemical reaction atoms are neither created or destroyed, only rearranged. b. All of the matter present in the reactants is also present in the products of the reaction. ...
physics/0010052 PDF
... front of α, β and γ one proceeds as following. Let dy and dz=0. Let df<0, if dx<0 then +α. The signs before β and γ are found analogically. Let's consider an exothermic and an isothermic reaction. Let's suppose that ∆V=0. In this occasion the 1st law of thermodynamics will be the following one: ∆U= ...
... front of α, β and γ one proceeds as following. Let dy and dz=0. Let df<0, if dx<0 then +α. The signs before β and γ are found analogically. Let's consider an exothermic and an isothermic reaction. Let's suppose that ∆V=0. In this occasion the 1st law of thermodynamics will be the following one: ∆U= ...
Thermodynamics Test Study Guide—AP _____ 1. The entropy
... beneath each species, what is the standard free energy change for this reaction at 298 K? C3H8(g) --------> CH4(g) ...
... beneath each species, what is the standard free energy change for this reaction at 298 K? C3H8(g) --------> CH4(g) ...
Ch 8 Notes: Chemical Equations and Reactions
... Use the “Solubility Rules” handout (at end of notes) to determine the solubility. If the compound is soluble that means that it will remain as ions in the solution, if it is insoluble then the compound precipitated out of the reaction (it became the precipitate or solid). 2. If at least one INSOLUBL ...
... Use the “Solubility Rules” handout (at end of notes) to determine the solubility. If the compound is soluble that means that it will remain as ions in the solution, if it is insoluble then the compound precipitated out of the reaction (it became the precipitate or solid). 2. If at least one INSOLUBL ...
CHEM 13 NEWS EXAM 1998 - University of Waterloo
... 22. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 grams of hydrogen and the other holds 8.0 grams of oxygen. Which one of the following statements regarding these gas samples is false? (The relative atomic mass of oxygen is 16.0 and that of hydrogen is 1. ...
... 22. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 grams of hydrogen and the other holds 8.0 grams of oxygen. Which one of the following statements regarding these gas samples is false? (The relative atomic mass of oxygen is 16.0 and that of hydrogen is 1. ...
File
... natural polymers like proteins and DNA Produced by condensation polymerization reactions. These reactions involve the formation of a small molecule (such as H2O, NH3, or HCl) The small molecule is said to be “condensed out” of the reaction. The monomer molecules bond at the site where atoms are remo ...
... natural polymers like proteins and DNA Produced by condensation polymerization reactions. These reactions involve the formation of a small molecule (such as H2O, NH3, or HCl) The small molecule is said to be “condensed out” of the reaction. The monomer molecules bond at the site where atoms are remo ...
Name___________________________________ Physical
... a. Name the more positive element (the first one) first. If there are more than one atom of this element, put the correct Greek prefix before the name. b. Name the more negative element (the last one) last. Change its name to end in -ide. If there are more than one atom of this element, put the corr ...
... a. Name the more positive element (the first one) first. If there are more than one atom of this element, put the correct Greek prefix before the name. b. Name the more negative element (the last one) last. Change its name to end in -ide. If there are more than one atom of this element, put the corr ...
Ch. 7 & 8 Notes (Chemical Reactions) teacher
... The coefficients represent either the number of _________ molecules present. liters if the substances are The coefficients can also represent _________ gases. ...
... The coefficients represent either the number of _________ molecules present. liters if the substances are The coefficients can also represent _________ gases. ...
SOME BASIC CHEMICAL TERMS
... One major goal of chemistry is to describe the properties of the many different forms of matter we encounter. Matter, the material of which the universe is composed, may be defined as anything that occupies space and has mass. Most of the materials we encounter in our daily lives, such as air, milk, ...
... One major goal of chemistry is to describe the properties of the many different forms of matter we encounter. Matter, the material of which the universe is composed, may be defined as anything that occupies space and has mass. Most of the materials we encounter in our daily lives, such as air, milk, ...
+ H 2 O(g)
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
Fall.2008.Week9.Lesson.2 - reich
... • 2 things come together to make 1 thing. • Carbon and Hydrogen react to form the compound methane. • C + H2 CH4 is the skeleton equation • C + 2H2 CH4 is the balanced equation ...
... • 2 things come together to make 1 thing. • Carbon and Hydrogen react to form the compound methane. • C + H2 CH4 is the skeleton equation • C + 2H2 CH4 is the balanced equation ...
Reaction Rate Reading Packet
... For the same mass, many small particles have a greater total surface area than one large particle. For example, steel wool has a larger surface area than a block of steel of the same mass. This allows oxygen molecules to collide with many more iron atoms per unit of time. The more surface contact be ...
... For the same mass, many small particles have a greater total surface area than one large particle. For example, steel wool has a larger surface area than a block of steel of the same mass. This allows oxygen molecules to collide with many more iron atoms per unit of time. The more surface contact be ...