BONUS: Which line in the above graph represents G for the reaction
... The velocities of product and reactant molecules are identical. ...
... The velocities of product and reactant molecules are identical. ...
reactions taking place within cells
... Organic chemistry The study of the chemistry of carbon compounds Homologous series A set of compounds with • similar chemical properties • same functional groups • a general formula where successive members differ by CH2 Structural formula How the various atoms are bonded to one another within the ...
... Organic chemistry The study of the chemistry of carbon compounds Homologous series A set of compounds with • similar chemical properties • same functional groups • a general formula where successive members differ by CH2 Structural formula How the various atoms are bonded to one another within the ...
ACS Practice Test 1
... 19. A given amount of electric charge deposits 2.159 g of silver from an Ag+ solution. What mass of copper from a Cu2+ solution will be deposited by the same quantity of electric charge? Atomic Molar Masses Ag 107.9 g·mol–1 Cu 63.5 g·mol–1 (A) 0.635 g (B) 1.97 g (C) 2.54 g (D) 127 g 20. If each of t ...
... 19. A given amount of electric charge deposits 2.159 g of silver from an Ag+ solution. What mass of copper from a Cu2+ solution will be deposited by the same quantity of electric charge? Atomic Molar Masses Ag 107.9 g·mol–1 Cu 63.5 g·mol–1 (A) 0.635 g (B) 1.97 g (C) 2.54 g (D) 127 g 20. If each of t ...
GC97F Pretest A - American Chemical Society
... (C) A catalyst provides additional energy to a reactant so it can achieve the necessary activation energy. (D) A catalyst provides an alternative reaction pathway with a lower activation energy. Questions 31 and 32 should be answered with reference to the equation 2 C(s) + O2(g) 2 CO(g) ∆H < 0 31. W ...
... (C) A catalyst provides additional energy to a reactant so it can achieve the necessary activation energy. (D) A catalyst provides an alternative reaction pathway with a lower activation energy. Questions 31 and 32 should be answered with reference to the equation 2 C(s) + O2(g) 2 CO(g) ∆H < 0 31. W ...
AGE article for Sept 2013
... Being an exothermic redox reaction, electrical energy can be obtained directly from chemical energy if the two half reactions can be separated, with oxidation of hydrogen occurring at an anode and reduction of oxygen at a cathode. The fuel cell can operate with either an acid or an alkaline electrol ...
... Being an exothermic redox reaction, electrical energy can be obtained directly from chemical energy if the two half reactions can be separated, with oxidation of hydrogen occurring at an anode and reduction of oxygen at a cathode. The fuel cell can operate with either an acid or an alkaline electrol ...
Chemistry EOC Review
... 59) You want to dissolve as much carbon dioxide as possible into a 2 liter bottle of Mountain Dew. What conditions of temperature (high or low) and pressure (high or low) should you use to dissolve the maximum amount of CO2? Would your answers be the same if you were trying to dissolve as much sugar ...
... 59) You want to dissolve as much carbon dioxide as possible into a 2 liter bottle of Mountain Dew. What conditions of temperature (high or low) and pressure (high or low) should you use to dissolve the maximum amount of CO2? Would your answers be the same if you were trying to dissolve as much sugar ...
School of Chemistry
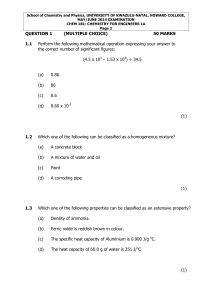
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
Net Ionic Prep Session NMSI INSTRUCTOR
... WRITE ALL WEAK ACIDS AND BASES AS MOLECULES—be on the look out for BF3 and its cousins BCl3, etc. They are classic Lewis acids and when reacting with ammonia (a classic weak Lewis base), the product is F3BNH3 (just smash everything together) since nitrogen donated its unshared electron pair to boron ...
... WRITE ALL WEAK ACIDS AND BASES AS MOLECULES—be on the look out for BF3 and its cousins BCl3, etc. They are classic Lewis acids and when reacting with ammonia (a classic weak Lewis base), the product is F3BNH3 (just smash everything together) since nitrogen donated its unshared electron pair to boron ...
Name: 1) At 1 atmosphere and 298 K, 1 mole of H O(l) molecules
... Careful observation reveals that these bubbles rise to the surface because CO2 gas is much less dense than water. However, not all of the CO2 gas rises to the surface; some of it dissolves in the water. The dissolved CO2 can react with water to form carbonic acid, H2 CO3 . ...
... Careful observation reveals that these bubbles rise to the surface because CO2 gas is much less dense than water. However, not all of the CO2 gas rises to the surface; some of it dissolves in the water. The dissolved CO2 can react with water to form carbonic acid, H2 CO3 . ...
Thermochemistry
... The apparatus most often used for calorimetric measurements is a calorimeter – in your lab this was simply polystyrene coffee cup (see slide). Calorimeter: “Device used to measure the heat absorbed or evolved during chemical or physical change” Discussion: Can any container be used as a calorimeter? ...
... The apparatus most often used for calorimetric measurements is a calorimeter – in your lab this was simply polystyrene coffee cup (see slide). Calorimeter: “Device used to measure the heat absorbed or evolved during chemical or physical change” Discussion: Can any container be used as a calorimeter? ...
31 BIOMOLECULES Y MODULE - 7
... You are aware that our body, plants and other animals are made up of many chemical substances. There are certain complex organic molecules which form the basis of life. These build up living organisms and are also required for their growth and maintenance. Such molecules are called biomolecules. The ...
... You are aware that our body, plants and other animals are made up of many chemical substances. There are certain complex organic molecules which form the basis of life. These build up living organisms and are also required for their growth and maintenance. Such molecules are called biomolecules. The ...
Because the iron sheet is denser than the iron powder. Because
... to indicate error in the experiment determine the method of an experiment ...
... to indicate error in the experiment determine the method of an experiment ...
Untitled
... The [H3O+] of any aqueous solution is a very important characteristic, and we often need to talk about it. It is inconvenient to talk about the concentration in units such as 4.50 x 10-12 M or numbers similar to this form. So scientist defined a new number called _____ to talk about the concentratio ...
... The [H3O+] of any aqueous solution is a very important characteristic, and we often need to talk about it. It is inconvenient to talk about the concentration in units such as 4.50 x 10-12 M or numbers similar to this form. So scientist defined a new number called _____ to talk about the concentratio ...
Document
... Which statement is true if 12 moles of CO and 12 moles of Fe2O3 are allowed to react? 3CO(g) + Fe2O3(s) 2Fe(s) + 3CO2(g) a. The limiting reactant is CO and 8.0 mol Fe will be formed. b. The limiting reactant is Fe2O3 and 24 mol Fe will be formed. c. The limiting reactant is CO and 3.0 mol CO2 will ...
... Which statement is true if 12 moles of CO and 12 moles of Fe2O3 are allowed to react? 3CO(g) + Fe2O3(s) 2Fe(s) + 3CO2(g) a. The limiting reactant is CO and 8.0 mol Fe will be formed. b. The limiting reactant is Fe2O3 and 24 mol Fe will be formed. c. The limiting reactant is CO and 3.0 mol CO2 will ...
Sherbert
... Chemical reactions, including combustion and the reactions of acids, are important in both nonliving and living systems and involve energy transfer ...
... Chemical reactions, including combustion and the reactions of acids, are important in both nonliving and living systems and involve energy transfer ...
GQ2613291336
... calculated. The corresponding halogenated 1,2thiazine has been identified as a product of halogenation. A suitable reaction scheme is proposed and an appropriate rate law is deduced to account for the observed kinetic and thermodynamic data. Keywords – 1,2-thiazine, Bromine (Br2), Conductivity, rate ...
... calculated. The corresponding halogenated 1,2thiazine has been identified as a product of halogenation. A suitable reaction scheme is proposed and an appropriate rate law is deduced to account for the observed kinetic and thermodynamic data. Keywords – 1,2-thiazine, Bromine (Br2), Conductivity, rate ...