Chapter 5: Gases - HCC Learning Web
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
Homework 5-7 answers
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
... C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C. A) 1.97 10–5 J B) 1.0 10–2 J C) 329 J D) 7.51 kJ E) 10.5 kJ Ans: D Category: Medium Section: 6.5 ...
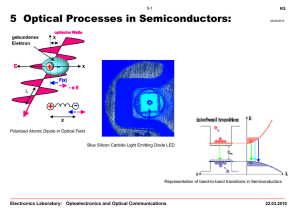
Chapter 5: Optical Processes in Semiconductors
... • Nonclassical description of the motion of bound electrons in the static force fields of the atomic nucleus or crystal lattice and a oscillating light wave • The equation of motion and polarization has to be described in a quantized 2-energy level material system by a quantum mechanical model based ...
... • Nonclassical description of the motion of bound electrons in the static force fields of the atomic nucleus or crystal lattice and a oscillating light wave • The equation of motion and polarization has to be described in a quantized 2-energy level material system by a quantum mechanical model based ...
Regents Review Live
... 65.39 amu. What is the most common isotope of Zinc? Zn-65! What are the most common isotopes of: ...
... 65.39 amu. What is the most common isotope of Zinc? Zn-65! What are the most common isotopes of: ...
Carbon nanotubes - Duke CS
... automated production of microchips from silicon on which they are so sharp, the nanotubes emit electrons at lower voltthe computer industry is built. ages than electrodes made from most other materials, and Before we can think about making more complex, nano- their strong carbon bonds allow nanotube ...
... automated production of microchips from silicon on which they are so sharp, the nanotubes emit electrons at lower voltthe computer industry is built. ages than electrodes made from most other materials, and Before we can think about making more complex, nano- their strong carbon bonds allow nanotube ...
CO2 Dissociation using the Versatile Atmospheric Dielectric Barrier
... carbon sequestration [8]. Even so, large amounts of research have and are currently being done to overcome these issues. ...
... carbon sequestration [8]. Even so, large amounts of research have and are currently being done to overcome these issues. ...
Midterm Review
... d. that in a copper penny, there is one atom for every person on Earth ____ 55. The range in size of most atomic radii is approximately ____. a. 2 to 5 cm c. 5 10 m to 2 b. 2 to 5 nm d. 5 10 m to 2 ...
... d. that in a copper penny, there is one atom for every person on Earth ____ 55. The range in size of most atomic radii is approximately ____. a. 2 to 5 cm c. 5 10 m to 2 b. 2 to 5 nm d. 5 10 m to 2 ...
Orthogonal metals: The simplest non-Fermi liquids
... to a Slater-Jastrow wave function, but with a Jastrow factor replaced by a different function. We then turn our attention to the phase transition, and explain that the transition between the two metallic phases is marked by a change in the electron spectral function. We show that in a mean-field appr ...
... to a Slater-Jastrow wave function, but with a Jastrow factor replaced by a different function. We then turn our attention to the phase transition, and explain that the transition between the two metallic phases is marked by a change in the electron spectral function. We show that in a mean-field appr ...
Semiclassical Green`s functions and an instanton formulation of
... where in both cases the Born-Oppenheimer approximation is first applied to obtain a single-surface Hamiltonian. The Im F method32 can be used to derive the instanton approximation to the adiabatic escape rate from metastable states in the high- or low-temperature limit26 although its application to ...
... where in both cases the Born-Oppenheimer approximation is first applied to obtain a single-surface Hamiltonian. The Im F method32 can be used to derive the instanton approximation to the adiabatic escape rate from metastable states in the high- or low-temperature limit26 although its application to ...
Optimisation of Quantum Trajectories Driven by Strong-Field
... which is only one example of what can be achieved with cycle-shaped high-field light-waves, has farreaching implications for attosecond spectroscopy and molecular self-probing. Controlling the shape of the driving laser waveform allows direct steering of the quasi-free electron trajectories at the h ...
... which is only one example of what can be achieved with cycle-shaped high-field light-waves, has farreaching implications for attosecond spectroscopy and molecular self-probing. Controlling the shape of the driving laser waveform allows direct steering of the quasi-free electron trajectories at the h ...
Theoretical Study of Carrier Capture into Semiconductor Quantum
... this work can be summarized as follows: 1. As will be shown later on (Chap. 3), the hole capture cannot be viewed as a classical diffusion process and using of the constant hole capture time in the calculations of Fig. 1.9 is not justified. In the light of this finding the agreement between the calc ...
... this work can be summarized as follows: 1. As will be shown later on (Chap. 3), the hole capture cannot be viewed as a classical diffusion process and using of the constant hole capture time in the calculations of Fig. 1.9 is not justified. In the light of this finding the agreement between the calc ...
X-Ray Diffraction and Scanning Probe Microscopy
... arrays of dots or lines on a 35-mm slide are spaced by many thousands of angstroms. Making these two modifications again leads to diffraction, as the wavelength and feature spacing are sufficiently close in scale to create observable effects. Thus, when these slides are used to view point sources of ...
... arrays of dots or lines on a 35-mm slide are spaced by many thousands of angstroms. Making these two modifications again leads to diffraction, as the wavelength and feature spacing are sufficiently close in scale to create observable effects. Thus, when these slides are used to view point sources of ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.