Matter Vocab Part 4
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
Modules to examine on the Arrangement of Electrons in Atoms website
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
[30 pts] While the spins of the two electrons in a hydrog
... 1. [30 pts] While the spins of the two electrons in a hydrogen molecule must be antiparallel in the the ground state, there is a degeneracy due to the spin of the two protons, s1 = 12 and s2 = 21 . a) Why is the Pauli exclusion priciple apparently violated for the protons? ie. why can the protons ha ...
... 1. [30 pts] While the spins of the two electrons in a hydrogen molecule must be antiparallel in the the ground state, there is a degeneracy due to the spin of the two protons, s1 = 12 and s2 = 21 . a) Why is the Pauli exclusion priciple apparently violated for the protons? ie. why can the protons ha ...
to as MS Word file
... 5. If Aˆ and Bˆ are Hermitian operators and c1 and c2 are real constants, then c1 Aˆ + c2 Bˆ must be a Hermitian operator. 6. It is possible to measure p2 (the square of the magnitude of the momentum) and the energy simultaneously to arbitrary accuracy for a particle in a box. 7. The maximum probabi ...
... 5. If Aˆ and Bˆ are Hermitian operators and c1 and c2 are real constants, then c1 Aˆ + c2 Bˆ must be a Hermitian operator. 6. It is possible to measure p2 (the square of the magnitude of the momentum) and the energy simultaneously to arbitrary accuracy for a particle in a box. 7. The maximum probabi ...
Lecture notes in Solid State 3 Eytan Grosfeld
... electronic charge. The conductivity is directly proportional to the electronic density n; and, to τ , the mean free time between collisions of the conducting electrons with defects that are generically present in the system: • Static defects that scatter the electron elastically, including: static i ...
... electronic charge. The conductivity is directly proportional to the electronic density n; and, to τ , the mean free time between collisions of the conducting electrons with defects that are generically present in the system: • Static defects that scatter the electron elastically, including: static i ...
Exam #2
... mass of the nucleus is concentrated in a very small volume. The electron diffraction experiment demonstrated Heisenberg’s hypothesis that matter and energy are interconvertable. The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electro ...
... mass of the nucleus is concentrated in a very small volume. The electron diffraction experiment demonstrated Heisenberg’s hypothesis that matter and energy are interconvertable. The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electro ...
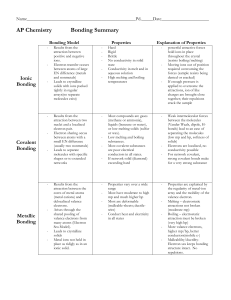
Types of Bonding Summary
... valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
... valence electrons from many atoms (Electron Sea Model). Leads to crystalline solids Metal ions not held in place as ridigly as in an ionic solid. ...
The Quantum Model of the Atom
... -symbolized by l, indicates the shape of the orbital -known as a sublevel -the # of orbital shapes possible is equal to n -values are zero and positive integers less than or equal to n-1 (0 = s, 1 = p, 2 = d, 3 = f) -s orbitals are spherical; p orbitals are dumbbell shaped; d and f orbitals are more ...
... -symbolized by l, indicates the shape of the orbital -known as a sublevel -the # of orbital shapes possible is equal to n -values are zero and positive integers less than or equal to n-1 (0 = s, 1 = p, 2 = d, 3 = f) -s orbitals are spherical; p orbitals are dumbbell shaped; d and f orbitals are more ...
m ι
... intensity—the larger the amplitude, the brighter the light. • Wavelength (l, lambda): a measure of the distance covered by the wave. • Frequency (n, nu): the number of waves that pass a point in a given period of time. Unit = cycles/s or s–1 (Hz, hertz). ...
... intensity—the larger the amplitude, the brighter the light. • Wavelength (l, lambda): a measure of the distance covered by the wave. • Frequency (n, nu): the number of waves that pass a point in a given period of time. Unit = cycles/s or s–1 (Hz, hertz). ...
Combining and Choosing Analytical Techniques
... Positive ions are formed in the ionisation chamber when an electron beam dislodges electrons from the sample atoms The positive ions are accelerated by an electric field The ions enter a magnetic field perpendicular to their path. This causes the ions to move in a curved path with a radius that depe ...
... Positive ions are formed in the ionisation chamber when an electron beam dislodges electrons from the sample atoms The positive ions are accelerated by an electric field The ions enter a magnetic field perpendicular to their path. This causes the ions to move in a curved path with a radius that depe ...
The Quantum Numbers
... The second quantum number ( l) is the angular momentum quantum number and describes the shape or type of orbital. Within an energy level there are four known possible sub energy levels each with a characteristic shape. The value of the sub energy levels can be 0 to (n-1). However, it is a common pra ...
... The second quantum number ( l) is the angular momentum quantum number and describes the shape or type of orbital. Within an energy level there are four known possible sub energy levels each with a characteristic shape. The value of the sub energy levels can be 0 to (n-1). However, it is a common pra ...
Worksheet
... 1) Summarize the contributions of each of the following individuals to our understanding of the atom and atomic structure (you may have to look back into CH 4 for the first 3…or the summary on page 133). Include a sketch of what the atom would look like according to their explanations. • Dalton: ...
... 1) Summarize the contributions of each of the following individuals to our understanding of the atom and atomic structure (you may have to look back into CH 4 for the first 3…or the summary on page 133). Include a sketch of what the atom would look like according to their explanations. • Dalton: ...
Physics 1020 Ch 10-12 Practice Exam (2).
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.






![[30 pts] While the spins of the two electrons in a hydrog](http://s1.studyres.com/store/data/002487557_1-ac2bceae20801496c3356a8afebed991-300x300.png)