* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ppt
Tight binding wikipedia , lookup
Wave–particle duality wikipedia , lookup
Hydrogen atom wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron configuration wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
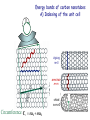
Electrons in Solids Carbon as Example • Electrons are characterized by quantum numbers which can be measured accurately, despite the uncertainty relation. In a solid these quantum numbers are: Energy: E Momentum: px,y,z • E is related to the translation symmetry in time (t), px,y,z to the translation symmetry in space (x,y,z) . • Symmetry in time allows t = E = 0 (from E · t ≥ h/4 ) Symmetry in space allows x = p = 0 (from p · x ≥ h/4 ) • The quantum numbers px,y,z live in reciprocal space since p = ћ k . Likewise, the energy E corresponds to reciprocal time. Therefore, one needs to think in reciprocal space-time, where large and small are inverted (see Lecture 6 on diffraction). Use a single crystal to simplify calculations Unit cell Instead of calculating the electrons for an infinite crystal, consider just one unit cell (which is equivalent to a molecule). Then add the couplings to neighbor cells via hopping energies. The unit cell in reciprocal space is called the Brillouin zone. Two-dimensional energy bands of graphene E Empty EFermi Occupied K Empty =0 kx,y M K M Occupied In two dimensions one has the quantum numbers E, px,y . Energy band dispersions (or simply energy bands) plot E vertical and kx , ky horizontal. Energy bands of graphite (including bands) E The graphite energy bands resemble those of graphene, but the , * bands broaden due to the interaction between the graphite layers (via the pz orbitals). * * pz EFermi px,y pz sp2 s kx,y Brillouin zone M K Energy bands of carbon nanotubes A) Indexing of the unit cell zigzag m=0 armchair n=m chiral nm0 Circumference Cr na1 ma2 Energy bands of carbon nanotubes B) Quantization along the circumference Analogous to Bohr’s quantization condition one requires that an integer number n of electron wavelengths fits around the circumference of the nanotube. (Otherwise the electron waves would interfere destructively.) This leads to a discrete number of allowed wavelengths n and k values kn = 2/n . (Compare the quantization condition for a quantum well, Lect. 2, Slide 9). Two-dimensional k-space gets transformed into a set of onedimensional k-lines (see next). k (A) Wrapping vectors (red) and allowed wave vectors kn (purple) for (3,0) zigzag, (3,3) armchair, and (4,2) chiral nanotubes. If the metallic K-point lies on a purple line, the nanotube is metallic, e.g. for (3,0) and (3,3). The (4,2) nanotube does not contain K, so it has a band gap. All armchair nanotubes (n,n) are metallic, since the purple line through contains the two orange K-points. Note that the purple lines are always parallel to the axis of the nanotube, since the quantization occurs in the perpendicular direction around the circumference. (B) Band structure of a (6,6) armchair nanotube, including the metallic K-point (orange dot). Each band corresponds to a purple quantization line. Their spacing is Δk = 2 / 1 = 2 / circumference = 1/ radius . Energy bands of carbon nanotubes C) Relation to graphene (5,5) Nanotube folded in half Two steps lead from the bands of graphene to those of a carbon nanotube: Graphene 1) The gray continuum of 2D bands gets quantized into discrete 1D bands. 2) The unit cell and Brillouin zone need to be converted from hexagonal (graphene) to rectangular (armchair nanotube. Thereby, energy bands become back-folded. EF K M (for the dashed band) Two classes of solids Carbon nanotubes cover both Energy Metals empty levels • Energy levels are continuous . • Electrons need very little energy to move electrical conductor filled levels Semiconductors, Insulators empty levels • Filled and empty energy levels separated by an energy gap. • Electrons need a lot of energy to move poor conductor . are gap filled levels Measuring the quantum numbers of electrons in a solid The quantum numbers E and k can both be measured by angle-resolved photoemission. This is an elaborate use of the photoelectric effect which was explained as quantum phenomenon by Einstein : Photon in Electron outside Electron inside Energy and momentum of an emitted photoelectron are measured. Use energy conservation to get the electron energy: Electron energy outside the solid − Photon energy = Electron energy inside the solid Energy bands of graphene from photoemission Evolution of the in-plane band dispersion with the number of layers In-plane k-components (single layer graphene) Evolution of the perpendicular band dispersion with the number of layers: N monolayers produce N discrete k-points. Ohta et al., Phys. Rev. Lett, 98, 206802 (2007) The density of states D(E) D(E) is defined as the number of states per energy interval. Each electron with a distinct wave function counts as a state. D(E) involves a summation over k, so the k-information is thrown out. While energy bands can only be determined directly by angle-resolved photoemission, there are many techniques available for determining the density of states. By going to low dimensions in nanostructures one can enhance the density of states at the edge of a band (E0). Such “van Hove singularities” can trigger interesting phenomena, such as superconductivity and magnetism. Tailoring the Density of States by Confinement to Density of States Nanostructures Adjust via d Potential Well 1D 3D EFermi d Energy Density of states of a single nanotube from scanning tunneling spectroscopy Calculated Density of States Scanning Tunneling Spectroscopy (STS) Cees Dekker, Physics Today, May 1999, p. 22. Optical spectra of nanotubes with different diameter, chirality Simultaneous data for fluorescence (x-axis) and absorption (y-axis) identify the nanotubes completely. Bachilo et al., Science 298, 2361 (2002) Need to prevent nanotubes from touching each other for sharp levels Sodium Dodecyl Sulfate (SDS) O'Connell et al, Science 297, 593 (2002) Quantized Conductance Dip nanotubes into a liquid metal (mercury, gallium). Each time an extra nanotube reaches the metal. the conductance increases by the same amount. The conductance quantum: G0 = 2 e2/h 1 / 13k (Factor of 2 for spin , ) Each wave function = band = channel contributes G0 . Expect 2G0 = 4 e2/h for nanotubes, since 2 bands cross EF at the K-point. This is indeed observed for better contacted nanotubes (Kong et al., Phys. Rev. Lett. 87, 106801 (2001)). Cees Dekker, Physics Today, May 1999, p. 22. Limits of Electronics from Information Theory Conductance per channel: G = G0•T Energy to switch one bit: E = kBT • ln2 Time to switch one bit: t = h/E Energy to transport a bit: E = kBT • d/c (G0 = 2 e2/h, transmission T1) (distance d, frequency ) Birnbaum and Williams, Physics Today, Jan. 2000, p. 38. Landauer, Feynman Lectures on Computation . Energy scales in carbon nanotubes ~20 eV Band width ( + * band) ~ 1 eV Quantization along the circumference ( k = 1/r ) ~ 0.1 eV Coulomb blockade (charging energy ECoul = Q/eC ) Quantization energy along the axis ( k|| = 2/L ) ~ 0.001 eV Many-electron effects (electron holon + spinon)