Test - Regents
... 15 Which formula is correct for ammonium sulfate? (3) NH4(SO4)2 (1) NH4SO4 (2) (NH4)2SO4 (4) (NH4)2(SO4)2 16 An example of an empirical formula is (3) C2H4(OH)2 (1) CH4 (2) C2H4 (4) C6H12O6 ...
... 15 Which formula is correct for ammonium sulfate? (3) NH4(SO4)2 (1) NH4SO4 (2) (NH4)2SO4 (4) (NH4)2(SO4)2 16 An example of an empirical formula is (3) C2H4(OH)2 (1) CH4 (2) C2H4 (4) C6H12O6 ...
The Complete Notes - Joliet Junior College
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don’t get round to swinging a club – what do you think happens when you tee off for the first time? ...
document
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
Microsoft Word
... Redox reactions are characterized by a transfer of electrons. An atom is oxidized (loses electrons) if its oxidation number increases (becomes more positive) in a chemical reaction; an atom is reduced (gains electrons) if its oxidation number decreases. ...
... Redox reactions are characterized by a transfer of electrons. An atom is oxidized (loses electrons) if its oxidation number increases (becomes more positive) in a chemical reaction; an atom is reduced (gains electrons) if its oxidation number decreases. ...
Chemistry 101: The Complete Notes
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don‟t get round to swinging a club – what do you think happens when you tee off for the first time? ...
... remembering. An analogy would be this: you read all the books out there on the subject of golf, but don‟t get round to swinging a club – what do you think happens when you tee off for the first time? ...
valence electrons
... • A visual representation used by scientists to help show chemical bonds • Only involves valence electrons • Consists of the elements symbol, which represents the innermost electrons too, and the valence electrons surrounding it. • Example: Lithium has 3 electrons but only 1 valence----Li • The numb ...
... • A visual representation used by scientists to help show chemical bonds • Only involves valence electrons • Consists of the elements symbol, which represents the innermost electrons too, and the valence electrons surrounding it. • Example: Lithium has 3 electrons but only 1 valence----Li • The numb ...
full text - pdf 452 kB
... increasing temperature. Most isocoulombic reactions where an ion reacts with a neutral species have smaller absolute values of AC, and positive AS values (11,14). These negative values indicate that the water structure becomes more organized with the formation of the complex. One possible explanatio ...
... increasing temperature. Most isocoulombic reactions where an ion reacts with a neutral species have smaller absolute values of AC, and positive AS values (11,14). These negative values indicate that the water structure becomes more organized with the formation of the complex. One possible explanatio ...
Chemistry 11 Exam 1 Spring 2006 When answering questions be
... Which reactant was oxidized? Explain. Silver started with an oxidation number of 0 and ended with an oxidation number of +1. This represents a loss of an electron so the silver was oxidized. Which reactant was reduced? Explain. Nitrogen in the nitrate ion has an oxidation number of +5. The oxidation ...
... Which reactant was oxidized? Explain. Silver started with an oxidation number of 0 and ended with an oxidation number of +1. This represents a loss of an electron so the silver was oxidized. Which reactant was reduced? Explain. Nitrogen in the nitrate ion has an oxidation number of +5. The oxidation ...
Chapter 6 Chemical Reactions and Change
... Note that when Mg and Fe reacted with oxygen, they lost electrons and became positively charged in the process; the loss of electrons is called oxidation. The oxygen gained electrons and this is called reduction. All elements in their elemental state are neutral and are assigned an oxidation state o ...
... Note that when Mg and Fe reacted with oxygen, they lost electrons and became positively charged in the process; the loss of electrons is called oxidation. The oxygen gained electrons and this is called reduction. All elements in their elemental state are neutral and are assigned an oxidation state o ...
Lecture 6
... Note that when Mg and Fe reacted with oxygen, they lost electrons and became positively charged in the process; the loss of electrons is called oxidation. The oxygen gained electrons and this is called reduction. All elements in their elemental state are neutral and are assigned an oxidation state o ...
... Note that when Mg and Fe reacted with oxygen, they lost electrons and became positively charged in the process; the loss of electrons is called oxidation. The oxygen gained electrons and this is called reduction. All elements in their elemental state are neutral and are assigned an oxidation state o ...
Class 8: Introduction to VSEPR Theory
... behavior of valence electrons in molecules, it does not provide information about the shapes of molecules. • Remember: Class #4 ▫ Lewis theory predicts water to be linear, however, the molecule is actually bent ▫ Distinct molecular shapes arise due to the distances between bonded atoms and the angle ...
... behavior of valence electrons in molecules, it does not provide information about the shapes of molecules. • Remember: Class #4 ▫ Lewis theory predicts water to be linear, however, the molecule is actually bent ▫ Distinct molecular shapes arise due to the distances between bonded atoms and the angle ...
Chemistry IGCSE Revision PDF File
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
Chapter 11 section 2 questions - the atom
... Electrons are the negatively charged particles found in the energy levels that surround the nucleus - this like the rings on a target! Electrons have a VERY small mass - almost inconsequential to the atomic mass. Electrons carry a negative charge and are held in place by the positively charged proto ...
... Electrons are the negatively charged particles found in the energy levels that surround the nucleus - this like the rings on a target! Electrons have a VERY small mass - almost inconsequential to the atomic mass. Electrons carry a negative charge and are held in place by the positively charged proto ...
Preview Sample 1
... A) there is the loss of one or more electrons from one atom to another atom of the same molecule. B) there is the gain of one or more electrons from one atom to another atom of the same molecule. C) one of the atoms has a greater affinity for electrons than the other atom of the same molecule. D) on ...
... A) there is the loss of one or more electrons from one atom to another atom of the same molecule. B) there is the gain of one or more electrons from one atom to another atom of the same molecule. C) one of the atoms has a greater affinity for electrons than the other atom of the same molecule. D) on ...
401
... within a single molecule. Actually, we can neglect some (large) parts of the antisymmetrization operations without affecting the accuracy (i.e., chemical accuracy) of the calculations: it would be a big loss if we have to assure antisymmetry for all electron pairs in a molecule. In this paper, we pr ...
... within a single molecule. Actually, we can neglect some (large) parts of the antisymmetrization operations without affecting the accuracy (i.e., chemical accuracy) of the calculations: it would be a big loss if we have to assure antisymmetry for all electron pairs in a molecule. In this paper, we pr ...
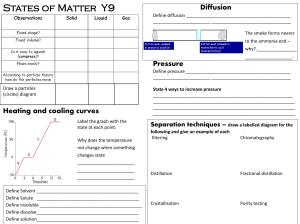
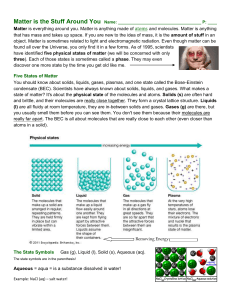
Stuff Matters Handout
... object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can be found all over the Universe, you only find it in a few forms. As of 1995, scientists have identified five physical states of matter (we will be concerned with only three). Each of those states is so ...
... object. Matter is sometimes related to light and electromagnetic radiation. Even though matter can be found all over the Universe, you only find it in a few forms. As of 1995, scientists have identified five physical states of matter (we will be concerned with only three). Each of those states is so ...
Document
... (b) Dividing each subscript by 5, we get the empirical formula CHN. (c) Because the subscripts in the formula for nitrous oxide are already the smallest possible whole numbers, its empirical formula is the same as its molecular formula N2O. Think About It Make sure that the ratio in each empirical f ...
... (b) Dividing each subscript by 5, we get the empirical formula CHN. (c) Because the subscripts in the formula for nitrous oxide are already the smallest possible whole numbers, its empirical formula is the same as its molecular formula N2O. Think About It Make sure that the ratio in each empirical f ...
Three-dimensional square water in the presence of an external
... In the case of fixed temperature and variable external electric field, we compare our results with those obtained by Suresh et al.8 for a theoretical model of water. The mean HB number for two different temperatures are shown in Fig. 4, where we used a constant value for the magnitude of the water d ...
... In the case of fixed temperature and variable external electric field, we compare our results with those obtained by Suresh et al.8 for a theoretical model of water. The mean HB number for two different temperatures are shown in Fig. 4, where we used a constant value for the magnitude of the water d ...
C4C5C6
... same number of shells Elements in the same group have the same number of electrons in the outer shell. They all have similar properties because they have the same number of electrons in the outer shell. ...
... same number of shells Elements in the same group have the same number of electrons in the outer shell. They all have similar properties because they have the same number of electrons in the outer shell. ...
College Chemistry 1 Note Guide(free download)
... 7. give a general overview of the periodic table and point out where types of elements and families/groups of elements are found. 8. introduce the concept of the mole roadmap and demonstrate how to use this concept in chemical calculations. 9. go through the expanded rules of nomenclature. 10. discu ...
... 7. give a general overview of the periodic table and point out where types of elements and families/groups of elements are found. 8. introduce the concept of the mole roadmap and demonstrate how to use this concept in chemical calculations. 9. go through the expanded rules of nomenclature. 10. discu ...
Assistant Professor Chemistry, Class-2, Advt No. 84/2016
... According to Pearson theory, a hard base is one whose donor atom has (A) high electronegativity, high polarizability and easy to oxidize (B) high electronegativity, low polarizability and difficult to oxidize (C) low electronegativity, lowpolarizability and difficult to oxidize (D) low electronegati ...
... According to Pearson theory, a hard base is one whose donor atom has (A) high electronegativity, high polarizability and easy to oxidize (B) high electronegativity, low polarizability and difficult to oxidize (C) low electronegativity, lowpolarizability and difficult to oxidize (D) low electronegati ...
Gmelin Tips and Reminders
... will not have a structure in the Gmelin database. If your search by structure brings up 0 hits, try the Search Fields or Search All Text functions • Salts are usually drawn in ionic form (i.e. no bonds between the ions) • Setting Free Sites: a) click on an atom to set free sites or, b) select many a ...
... will not have a structure in the Gmelin database. If your search by structure brings up 0 hits, try the Search Fields or Search All Text functions • Salts are usually drawn in ionic form (i.e. no bonds between the ions) • Setting Free Sites: a) click on an atom to set free sites or, b) select many a ...