1. Select the correct statement about subatomic particles. a
... a. 1.8L c. 2.0L b. 3.6L d. 2.4L 81. How many moles of glucose, C6H12O6, can be “burned” biologically when 10.0 moles of oxygen are available? a. 0.938 mol d. 60.0 mol b. 1.67 mol e. 301 mol c. 53.3 mol 82. Hydrogen gas can be produced by reacting aluminum with sulfuric acid. How many moles of sulfur ...
... a. 1.8L c. 2.0L b. 3.6L d. 2.4L 81. How many moles of glucose, C6H12O6, can be “burned” biologically when 10.0 moles of oxygen are available? a. 0.938 mol d. 60.0 mol b. 1.67 mol e. 301 mol c. 53.3 mol 82. Hydrogen gas can be produced by reacting aluminum with sulfuric acid. How many moles of sulfur ...
section_2_review_set
... 1. What is the claim to fame for the proton? 2. What is the claim to fame for the electron? 3. What is the claim to fame for the neutron? 4. What is the mass of each of the following particles?: proton; neutron; electron. 5. What is the charge for each of the following particles?: proton; neutron; e ...
... 1. What is the claim to fame for the proton? 2. What is the claim to fame for the electron? 3. What is the claim to fame for the neutron? 4. What is the mass of each of the following particles?: proton; neutron; electron. 5. What is the charge for each of the following particles?: proton; neutron; e ...
AP Chemistry Summer Assignment - 2015
... EX. Ca(OH)2(s) → CaO(s) + H2O(g) 3. Metallic chlorates, when heated, decompose into metallic chlorides and oxygen gas. EX. 2KClO3(s) → 2KCl(s) + 3O2(g) 4. Some acids, when heated, decompose into nonmetallic oxides and water. EX. H2SO4 → H2O(l) + SO3(g) 5. Some oxides, when heated, decompose to the e ...
... EX. Ca(OH)2(s) → CaO(s) + H2O(g) 3. Metallic chlorates, when heated, decompose into metallic chlorides and oxygen gas. EX. 2KClO3(s) → 2KCl(s) + 3O2(g) 4. Some acids, when heated, decompose into nonmetallic oxides and water. EX. H2SO4 → H2O(l) + SO3(g) 5. Some oxides, when heated, decompose to the e ...
Chemistry 199 - Oregon State chemistry
... Yes, water acts like a Lewis base—especially in transition metal chemistry. The lone pairs of electrons on the oxygen atom are donated to a metal ion to form a new bond. ...
... Yes, water acts like a Lewis base—especially in transition metal chemistry. The lone pairs of electrons on the oxygen atom are donated to a metal ion to form a new bond. ...
Chemical Reactions & Balancing Equations
... – same number of atoms of each type of element on each side What if it isn’t balanced already? ...
... – same number of atoms of each type of element on each side What if it isn’t balanced already? ...
File
... 11. What are the rows in the periodic table called and what do they show? periods 12. Using the periodic table, find the element that is found in group 2 period 3. ...
... 11. What are the rows in the periodic table called and what do they show? periods 12. Using the periodic table, find the element that is found in group 2 period 3. ...
Chapter 30 - The Chemical Basis of Animal Life
... atom, leaving a proton behind. As a result, the hydrogen atom gains a slight positive charge. The remaining proton is attracted to negatively charged atoms of, for example, oxygen in nearby molecules. When this happens, a weak attraction, called a hydrogen bond, forms. The hydrogen atom in one water ...
... atom, leaving a proton behind. As a result, the hydrogen atom gains a slight positive charge. The remaining proton is attracted to negatively charged atoms of, for example, oxygen in nearby molecules. When this happens, a weak attraction, called a hydrogen bond, forms. The hydrogen atom in one water ...
Final Exam Review Guide
... Indicate the correct word(s) to complete each sentence. ________ 1. Ionic bonds are formed between metals and non-metals ________ 2. Covalent bonds are formed between non-metals and other non-metals. ________ 3. Metals do not form bonds with other metals ________ 4. The transition metals lose electr ...
... Indicate the correct word(s) to complete each sentence. ________ 1. Ionic bonds are formed between metals and non-metals ________ 2. Covalent bonds are formed between non-metals and other non-metals. ________ 3. Metals do not form bonds with other metals ________ 4. The transition metals lose electr ...
Atomic Number, Atomic Mass
... Number of protons always equals number of electrons. The number of protons is the Atomic Number (Z) and defines the element. The Mass Number (A) is the total mass of the atom, i.e. number of protons (Z) + number of neutrons (N) ...
... Number of protons always equals number of electrons. The number of protons is the Atomic Number (Z) and defines the element. The Mass Number (A) is the total mass of the atom, i.e. number of protons (Z) + number of neutrons (N) ...
ap chemistry – 2013-2014
... AP CHEMISTRY – 2013-2014 Course Description: This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristic ...
... AP CHEMISTRY – 2013-2014 Course Description: This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. This course is structured around six big ideas that include: Structure of matter, properties of matter-characteristic ...
WEEKLIES ISSUE
... found bound into atomic nuclei because they are unstable in an unbound state; after about 15 minutes, the neutron decays into a proton and an electron. Neutrons can be used to maintain nuclear chain reactions that can generate electricity. The electron is a lepton—a type of fundamental particle—and ...
... found bound into atomic nuclei because they are unstable in an unbound state; after about 15 minutes, the neutron decays into a proton and an electron. Neutrons can be used to maintain nuclear chain reactions that can generate electricity. The electron is a lepton—a type of fundamental particle—and ...
Lecture 6 - TCD Chemistry
... – With opposite phase – one nodal plane – Interaction with p orbitals 3 ...
... – With opposite phase – one nodal plane – Interaction with p orbitals 3 ...
Optional Molecular Shapes Project – Due Monday 5/14/12 You will
... explain why/how each molecular shape occurs including: State the bond angle(s) according to the VSEPR Theory (2pts each shape). Provide an example substance for each shape (2pts each shape). Identify the number of shared electron pairs and lone electron pairs for each shape below (2pts each shape). ...
... explain why/how each molecular shape occurs including: State the bond angle(s) according to the VSEPR Theory (2pts each shape). Provide an example substance for each shape (2pts each shape). Identify the number of shared electron pairs and lone electron pairs for each shape below (2pts each shape). ...
Bonding Conceptually easier to view bonding if one considers
... Electron density is not symmetric about the internuclear axis ...
... Electron density is not symmetric about the internuclear axis ...
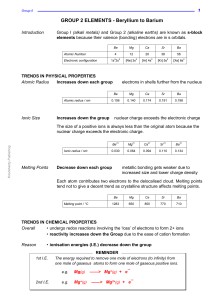
GROUP 2 ELEMENTS - Beryllium to Barium
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
... basic strength also increases down group this is because the solubility increases the metal ions get larger so charge density decreases there is a lower attraction between the OH¯ ions and larger dipositive ions the ions will split away from each other more easily there will be a greater concentrati ...
2 - FacultyWeb
... • Elements cannot be broken down by ordinary chemical means • Each element has unique physical and chemical properties: Physical properties are detectable with our senses, or are ...
... • Elements cannot be broken down by ordinary chemical means • Each element has unique physical and chemical properties: Physical properties are detectable with our senses, or are ...
UNIT NUM="1" ID="UN
... The chemical behavior of an atom is determined by the distribution of electrons in the atom’s electron shells. Beginning with hydrogen, the simplest atom, we can imagine building the atoms of the other elements by adding 1 proton and 1 electron at a time (along with an appropriate number of neutrons ...
... The chemical behavior of an atom is determined by the distribution of electrons in the atom’s electron shells. Beginning with hydrogen, the simplest atom, we can imagine building the atoms of the other elements by adding 1 proton and 1 electron at a time (along with an appropriate number of neutrons ...
Importance of Molecular Simulation for Studying Structural Properties
... computational techniques used to model or mimic the behavior of molecules. The techniques are used in the fields of computational chemistry, drug design, computational biology and materials science for studying molecular systems ranging from small chemical systems to large biological molecules and m ...
... computational techniques used to model or mimic the behavior of molecules. The techniques are used in the fields of computational chemistry, drug design, computational biology and materials science for studying molecular systems ranging from small chemical systems to large biological molecules and m ...
Atoms PowerPoint
... Occurs when atoms share electrons so that each atom can fill its valence shell some of the time Neither atom is strong enough to gain total control of any unpaired electrons There is an ongoing tug of war and these electrons remain attracted to both nuclei Occurs with atoms of 3, 4, 5, electrons in ...
... Occurs when atoms share electrons so that each atom can fill its valence shell some of the time Neither atom is strong enough to gain total control of any unpaired electrons There is an ongoing tug of war and these electrons remain attracted to both nuclei Occurs with atoms of 3, 4, 5, electrons in ...
Name
... 10. Name the three types of chemical reactions and give an example of each equation. Essential Standard 5b: The idea of atoms explains the conservation of matter: in chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. ...
... 10. Name the three types of chemical reactions and give an example of each equation. Essential Standard 5b: The idea of atoms explains the conservation of matter: in chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. ...
Cl Cl and
... 27. Why don’t elements of group 4 form ions of charge 4+? Why don’t they form ions of charge 4–? Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do eleme ...
... 27. Why don’t elements of group 4 form ions of charge 4+? Why don’t they form ions of charge 4–? Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do eleme ...