Problem 1: A brief history of life in the universe
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
... atoms escape more readily than nitrogen molecules even though the escape velocity is independent of the mass of the escaping object. The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, inter ...
Calculations on the equations reaction
... valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in ...
... valences this element can have in compounds? Write the formula of highest oxide of this element. 2. An element has serial number 19 define: а) charge of nucleus atom b) number of electrons c) number of neutrons and protons. Write electronic formula of element. What valences this element can have in ...
Document
... potential energy you gain. Generally speaking, chemistry does not concern itself with the potential energy from gravity. (b) Electrical - in certain materials, you can remove electrons from one area and send them to another. The area losing the electrons becomes more and ...
... potential energy you gain. Generally speaking, chemistry does not concern itself with the potential energy from gravity. (b) Electrical - in certain materials, you can remove electrons from one area and send them to another. The area losing the electrons becomes more and ...
Equilibrium and Pressure
... C. How does this value of Kp compare to the value you found before? ______________ ___________________________________________________________________ D. As the experiment reached equilibrium again, did the reactants or products increase? _____________________________________________________________ ...
... C. How does this value of Kp compare to the value you found before? ______________ ___________________________________________________________________ D. As the experiment reached equilibrium again, did the reactants or products increase? _____________________________________________________________ ...
Chapter 4 Notes
... Example: in NaH, the H is H-; in HCl, the H is H+. + + 2. The oxidation number of a free element is always 0. Example: The atoms in He and N2, for example, have oxidation numbers of 0. 3. The oxidation number of a monatomic ion equals the charge of the ion. Example: oxidation number of Na+ is +1; th ...
... Example: in NaH, the H is H-; in HCl, the H is H+. + + 2. The oxidation number of a free element is always 0. Example: The atoms in He and N2, for example, have oxidation numbers of 0. 3. The oxidation number of a monatomic ion equals the charge of the ion. Example: oxidation number of Na+ is +1; th ...
1994 Released Exam
... it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, or not at all in each set. Questions5-7 refer to the phasediagrambelow of a pure substance. ...
... it. Select the one letteredchoice that bestanswerseach questionor bestfits each statementand then fill in the correspondingoval on the answersheet.A choice may be used once, more than once, or not at all in each set. Questions5-7 refer to the phasediagrambelow of a pure substance. ...
Thermochemistry and calorimetry
... components of the calorimeter itself. It is therefore necessary to "calibrate" the calorimeter by measuring the temperature change that results from the introduction of a known quantity of heat. The resulting calorimeter constant, expressed in J K–1, can be regarded as the “heat capacity of the calo ...
... components of the calorimeter itself. It is therefore necessary to "calibrate" the calorimeter by measuring the temperature change that results from the introduction of a known quantity of heat. The resulting calorimeter constant, expressed in J K–1, can be regarded as the “heat capacity of the calo ...
CHEM 1405 Practice Exam #2
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
... A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) ...
Problem 5. Inorganic chains and rings
... agent)… The preparative procedure should be designed so as to avoid contamination by silicates or aluminates … Product B, in the form of well-crystallized sky-blue rods, remains stable at 0C if kept free of H2O and CO2… A solution of B in 50% potassium hydroxide turns grassy green upon heating or d ...
... agent)… The preparative procedure should be designed so as to avoid contamination by silicates or aluminates … Product B, in the form of well-crystallized sky-blue rods, remains stable at 0C if kept free of H2O and CO2… A solution of B in 50% potassium hydroxide turns grassy green upon heating or d ...
Module 2 Alcohols, halogenoalkanes and analysis
... In this module, you will study the physical properties and chemical reactions of two functional groups: alcohols and halogenoalkanes. The chemicals known as chlorofluorocarbons (CFCs) are halogenoalkanes. The image shows a satellite picture of the ozone hole (dark blue) over Antarctica in 2005; the o ...
... In this module, you will study the physical properties and chemical reactions of two functional groups: alcohols and halogenoalkanes. The chemicals known as chlorofluorocarbons (CFCs) are halogenoalkanes. The image shows a satellite picture of the ozone hole (dark blue) over Antarctica in 2005; the o ...
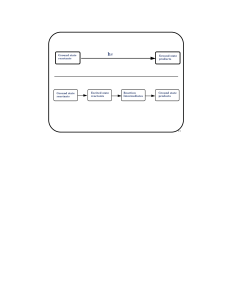
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.