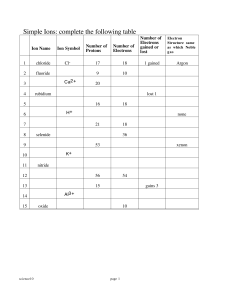
Lessons 9
... the total internal energy of a substance at a constant pressure chemists study the enthalpy change, ∆H, that accompanies a chemical process ∆H of a process is equivalent to its heat change at a constant pressure ∆H can be associated with physical changes as well as chemical reactions ...
... the total internal energy of a substance at a constant pressure chemists study the enthalpy change, ∆H, that accompanies a chemical process ∆H of a process is equivalent to its heat change at a constant pressure ∆H can be associated with physical changes as well as chemical reactions ...
Gr. 11 Chemistry Student Workbook (Spring 2016)
... One of the most important educational skills you can develop is how to monitor and track your own learning. Either at the end of class or at home, you will complete a daily entry in your learning log. Written Work: Use our marking scheme for daily class work (out of 5) to assess your written work. W ...
... One of the most important educational skills you can develop is how to monitor and track your own learning. Either at the end of class or at home, you will complete a daily entry in your learning log. Written Work: Use our marking scheme for daily class work (out of 5) to assess your written work. W ...
SUPPORT MATERIAL CLASS – X(science) FIRST TERM
... 1) Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 )Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3) Balanced Chemica ...
... 1) Chemical reaction— Chemical changes or chemical reactions are the changes in which one or more new substances are formed. 2 )Chemical Equations – Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3) Balanced Chemica ...
Thermochemistry Exam Review Questions
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
... an unknown solution. What is the pH for the unknown solution likely to be? A. 1.2 B. 3.0 C. 5.3 D. 9.0 12. What is the name of the ion when a positively charged proton combines with a water molecule? A. ammonium ion B. hydrogen ion C. hydronium ion D. hydroxide ion 13. What is the term for a substan ...
1984 Advanced Placement Exam
... 24. The formula for potassium hexacyanoferrate (II) 28. 2 A(g) + B(g) 2 C(g) is When the concentration of substance B in the re(A) K4[Fe(CN)6] (D) K2[Pt(CN)6] action above is doubled, all other factors being held constant, it is found that the rate of the reac(B) K3[Fe(CN)6] (E) KCN tion remains ...
... 24. The formula for potassium hexacyanoferrate (II) 28. 2 A(g) + B(g) 2 C(g) is When the concentration of substance B in the re(A) K4[Fe(CN)6] (D) K2[Pt(CN)6] action above is doubled, all other factors being held constant, it is found that the rate of the reac(B) K3[Fe(CN)6] (E) KCN tion remains ...
Chemistry HSC - The Bored of Studies Community
... and can be synthesised from many different hydrocarbons. Three ways: 1. Thermal cracking – requires very high temps and generally not used. End products hard to control since many places where bonds could break, early method. Accelerates reaction and drives equilibrium to reactants. 2. Catalytic cra ...
... and can be synthesised from many different hydrocarbons. Three ways: 1. Thermal cracking – requires very high temps and generally not used. End products hard to control since many places where bonds could break, early method. Accelerates reaction and drives equilibrium to reactants. 2. Catalytic cra ...
No Slide Title
... Weak acids and bases - Molecular compounds that are weak acids or weak bases are also weak electrolytes. Note that an acid forms H+ ion when added to water, and a base forms OH- ion. ...
... Weak acids and bases - Molecular compounds that are weak acids or weak bases are also weak electrolytes. Note that an acid forms H+ ion when added to water, and a base forms OH- ion. ...
CH 13
... “Mechanism” for the reaction of CO with NO2 at low temp NO2(g) + NO2(g) NO3(g) + NO(g) slow CO(g) + NO3(g) CO2(g) + NO2(g) fast CO(g) + NO2(g) NO(g) + CO2(g) overall The overall reaction, obtained by summing the individual steps is identical but the rate expressions are different. ...
... “Mechanism” for the reaction of CO with NO2 at low temp NO2(g) + NO2(g) NO3(g) + NO(g) slow CO(g) + NO3(g) CO2(g) + NO2(g) fast CO(g) + NO2(g) NO(g) + CO2(g) overall The overall reaction, obtained by summing the individual steps is identical but the rate expressions are different. ...
IB Chemistry HL Topic5 Questions 1. Which
... What are the signs of ∆Hο and ∆S ο for a reaction that is non-spontaneous at low temperature but spontaneous at high temperature? ...
... What are the signs of ∆Hο and ∆S ο for a reaction that is non-spontaneous at low temperature but spontaneous at high temperature? ...
Follow Along Notes - Jackson County School System
... Calculations involving equilibrium How to solve Equilibrium Problems: 1. Start with a balanced Chemical Equation 2. Write down the amounts (either concentration or pressure units) in an ICE table. 3. Shift the equilibrium by subtracting and adding x to either side to the equation. 4. Solve for x us ...
... Calculations involving equilibrium How to solve Equilibrium Problems: 1. Start with a balanced Chemical Equation 2. Write down the amounts (either concentration or pressure units) in an ICE table. 3. Shift the equilibrium by subtracting and adding x to either side to the equation. 4. Solve for x us ...
Student Review Packet
... NH4Cl(s) NH3(g) + HCl(g) for this reaction, ΔH = +42.1 kilocalories Suppose the substances in the reaction above are at equilibrium at 600 K in volume V and at pressure P. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is reestabl ...
... NH4Cl(s) NH3(g) + HCl(g) for this reaction, ΔH = +42.1 kilocalories Suppose the substances in the reaction above are at equilibrium at 600 K in volume V and at pressure P. State whether the partial pressure of NH3(g) will have increased, decreased, or remained the same when equilibrium is reestabl ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.