Thermodynamics: Entropy, Free Energy and the Direction of
... (e) Seawater in midwinter at 20C or in midsummer at 230C (f) 1mol of CF4(g) or 1mol of CCl4(g) PLAN: In general less ordered systems have higher entropy than ordered systems and entropy increases with an increase in temperature. SOLUTION: (a) 1mol of SO3(g) - more atoms ...
... (e) Seawater in midwinter at 20C or in midsummer at 230C (f) 1mol of CF4(g) or 1mol of CCl4(g) PLAN: In general less ordered systems have higher entropy than ordered systems and entropy increases with an increase in temperature. SOLUTION: (a) 1mol of SO3(g) - more atoms ...
2.0 Chem 20 Final Review
... • It is important to understand the difference between an ideal gas and a real gas.... – IDEAL GAS – does not really exist, it is hypothetical ...
... • It is important to understand the difference between an ideal gas and a real gas.... – IDEAL GAS – does not really exist, it is hypothetical ...
Lab # 18
... To balance equations, we follow these four rules: 1. Equations must be balanced so that the number of atoms of each element is equal on the left side (reactants) and on the right side (products) of the reaction. 2. We MUST NOT change the subscripts of any of the reactants or products; if we did that ...
... To balance equations, we follow these four rules: 1. Equations must be balanced so that the number of atoms of each element is equal on the left side (reactants) and on the right side (products) of the reaction. 2. We MUST NOT change the subscripts of any of the reactants or products; if we did that ...
Stoichiometry - VernonScienceLSA
... Problem What mass of CuO is required to make 10.0 g of Cu according to the reaction 2NH3 + 3CuO N2 + 3Cu + 3H2O if the reaction has a 58.0% yield? Solution (1) The actual yield is 10.0 g, we need to expect to produce more than 10.0 g of Cu since only 58.0% of what is expected will actually be prod ...
... Problem What mass of CuO is required to make 10.0 g of Cu according to the reaction 2NH3 + 3CuO N2 + 3Cu + 3H2O if the reaction has a 58.0% yield? Solution (1) The actual yield is 10.0 g, we need to expect to produce more than 10.0 g of Cu since only 58.0% of what is expected will actually be prod ...
DEPARTMENT OF CHEMISTRY, CFS, IIUM
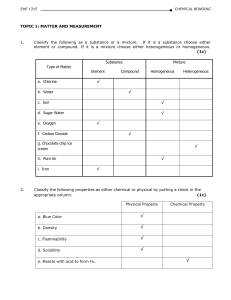
... producing new kinds of matter is called a physical property. A characteristic that depends on how a kind of matter changes suring interactions with other kinds of matter is called chemical property. Matter can also be classified according to the basic types of matter it contains. A simple substance ...
... producing new kinds of matter is called a physical property. A characteristic that depends on how a kind of matter changes suring interactions with other kinds of matter is called chemical property. Matter can also be classified according to the basic types of matter it contains. A simple substance ...
Review Unit: Chemistry Review
... Chemistry is primarily the study of changes in matter. For example, coals burning, fireworks exploding, and iron rusting are all changes studied in chemistry. A chemical change or chemical reaction is a change in which one or more new substances with different properties are formed. Chemistry also i ...
... Chemistry is primarily the study of changes in matter. For example, coals burning, fireworks exploding, and iron rusting are all changes studied in chemistry. A chemical change or chemical reaction is a change in which one or more new substances with different properties are formed. Chemistry also i ...
Worked solutions to textbook questions 1 Chapter 14 From organic
... C. There is a double bond between two carbon atoms in alkenes. The first members of the other homologous series, methane, methanol and methanoic acid all contain 1 carbon atom. Q2. The systematic name for CH3CH2CH2CH(CH3)2 is: A 1,1-dimethylbutane B 2-methylpentane C 2-methylpentene D propyldimethyl ...
... C. There is a double bond between two carbon atoms in alkenes. The first members of the other homologous series, methane, methanol and methanoic acid all contain 1 carbon atom. Q2. The systematic name for CH3CH2CH2CH(CH3)2 is: A 1,1-dimethylbutane B 2-methylpentane C 2-methylpentene D propyldimethyl ...
Chemistry - Tumkur University
... of stating II law of thermodynamics with respect to its spontaneity, spontaneous and nonspontaneous processes. Concept of entropy and its significance-illustrations for order, disorder, physical, chemical process and probability. Heat engine: Carnot’s cycle and derivation of the expression for its e ...
... of stating II law of thermodynamics with respect to its spontaneity, spontaneous and nonspontaneous processes. Concept of entropy and its significance-illustrations for order, disorder, physical, chemical process and probability. Heat engine: Carnot’s cycle and derivation of the expression for its e ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.