Chemistry
... 440 BC, the Greek philosopher Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made ...
... 440 BC, the Greek philosopher Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made ...
Chemical Properties - Michigan State University
... I think I’ll learn from incorrect answers. By seeing what they were confused on, I can revamp my next lesson to clear up any misconceptions. I think if students don’t understand the science behind chemical and physical changes, they wont really understand the concept. Knowing that chemical reactions ...
... I think I’ll learn from incorrect answers. By seeing what they were confused on, I can revamp my next lesson to clear up any misconceptions. I think if students don’t understand the science behind chemical and physical changes, they wont really understand the concept. Knowing that chemical reactions ...
word - My eCoach
... matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass o ...
... matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass o ...
Ch 16 Power Point
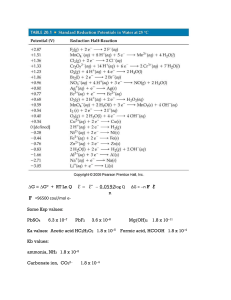
... 2H2(g) + O2(g) → 2H2O(g) + 483.6 kJ • The expression above is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat during the reaction as written. • Chemical coefficients in a thermochemical equation should be interpreted as numbers o ...
... 2H2(g) + O2(g) → 2H2O(g) + 483.6 kJ • The expression above is an example of a thermochemical equation, an equation that includes the quantity of energy released or absorbed as heat during the reaction as written. • Chemical coefficients in a thermochemical equation should be interpreted as numbers o ...
1. Which idea of John Dalton is no longer considered part of the
... In compliance with federal law, including the provisions of Title IX of the Education Amendments of 1972, the Department of Public Instruction does not discriminate on the basis of race, sex, religion, color, national or ethnic origin, age, disability, or military service in its policies, ...
... In compliance with federal law, including the provisions of Title IX of the Education Amendments of 1972, the Department of Public Instruction does not discriminate on the basis of race, sex, religion, color, national or ethnic origin, age, disability, or military service in its policies, ...
18-19 SpontEnt
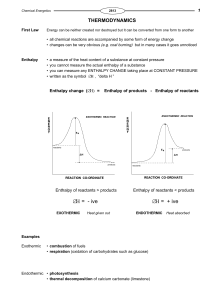
... Nature tends to move spontaneously from a state of lower probability to one of higher probability »!G.N. Lewis (Nobel Laureate) ...
... Nature tends to move spontaneously from a state of lower probability to one of higher probability »!G.N. Lewis (Nobel Laureate) ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... Other types of combination reactions include the combination of an element with a compound and the combination of two different compounds to form a new compound. For example, oxygen reacts with sulfur dioxide to make sulfur trioxide. 2 SO2(g) + O2(g) 2 SO3(g) Sulfur trioxide and water undergo a co ...
... Other types of combination reactions include the combination of an element with a compound and the combination of two different compounds to form a new compound. For example, oxygen reacts with sulfur dioxide to make sulfur trioxide. 2 SO2(g) + O2(g) 2 SO3(g) Sulfur trioxide and water undergo a co ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.