
Quarter 1
... C5.2C Draw pictures to distinguish the relationships between atoms in physical and chemical changes. ...
... C5.2C Draw pictures to distinguish the relationships between atoms in physical and chemical changes. ...
Unit 4 - Chemical Equilibrium
... Recognizing Equilibrium (pg 424) Gaseous Equilibrium : Consider the reaction : H2 (g) + I2 (g) ...
... Recognizing Equilibrium (pg 424) Gaseous Equilibrium : Consider the reaction : H2 (g) + I2 (g) ...
Second Year - WordPress.com
... found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mean of the other two elements of a triad. ...
... found to be approximately the mean of the other two elements of triad. b) Atomic weight of the middle element was found to be approximately the mean of the other two elements of a triad. c) Atomic number of any one element was found to be approximately the mean of the other two elements of a triad. ...
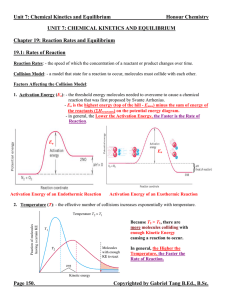
Unit 7 Reaction Rates and Equilibrium Notes
... does not participate in the forward or reverse reaction. b. Reducing the Volume will Drive the System TOWARDS the Side With LESS Gaseous Molecules. Since there are less space for the number of molecules, the system will have to shift to the side with lesser gaseous molecules to compensate. c. Conver ...
... does not participate in the forward or reverse reaction. b. Reducing the Volume will Drive the System TOWARDS the Side With LESS Gaseous Molecules. Since there are less space for the number of molecules, the system will have to shift to the side with lesser gaseous molecules to compensate. c. Conver ...
PURPOSE: To determine the value of the equilibrium constant for a
... Equilibrium results when the two reactions occur at equal rates. The ions are dissociating into solution at the same rate that the ions crystallize into the solid. The concentration of the ions in the solution remains constant. We may model this as the breaking and forming of the ionic bond between ...
... Equilibrium results when the two reactions occur at equal rates. The ions are dissociating into solution at the same rate that the ions crystallize into the solid. The concentration of the ions in the solution remains constant. We may model this as the breaking and forming of the ionic bond between ...
AP Chem unit 13 presentation
... 5. Define the change needed to reach equilibrium, and define the equilibrium concentrations by applying the change to the initial concentrations. 6. Substitute the equilibrium concentrations into the equilibrium expression, and solve for the unknown. 7. Check your calculated equilibrium concentratio ...
... 5. Define the change needed to reach equilibrium, and define the equilibrium concentrations by applying the change to the initial concentrations. 6. Substitute the equilibrium concentrations into the equilibrium expression, and solve for the unknown. 7. Check your calculated equilibrium concentratio ...
Amines - ncert
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
7. A timeline of symbols and signs in chemistry
... to be much in common with algebraic use of symbols and signs, there are important differences when used in chemical contexts. An understanding of how the present use came about may shed light on some of the problems that learners in schools may have with chemical equations. This article aims to expl ...
... to be much in common with algebraic use of symbols and signs, there are important differences when used in chemical contexts. An understanding of how the present use came about may shed light on some of the problems that learners in schools may have with chemical equations. This article aims to expl ...
Chapter 5: Thermochemistry
... → DH = negative Temperature decrease in calorimeter: → reaction is endothermic → DH = positive ...
... → DH = negative Temperature decrease in calorimeter: → reaction is endothermic → DH = positive ...
support material
... Avogadro Law (In 1811, Given by Avogadro) According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical ...
... Avogadro Law (In 1811, Given by Avogadro) According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.


















![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)



