Equilibrium - chemmybear.com
... (c) CO will decrease. A decrease in volume will result in an increase in pressure, the equilibrium will shift to the side with fewer gas molecules to decrease the pressure, , a shift to the left. (d) CO will remain the same. Once at equilibrium, the size of the solid will affect neither the reactio ...
... (c) CO will decrease. A decrease in volume will result in an increase in pressure, the equilibrium will shift to the side with fewer gas molecules to decrease the pressure, , a shift to the left. (d) CO will remain the same. Once at equilibrium, the size of the solid will affect neither the reactio ...
Unit 5: Chemical Kinetics and Equilibrium
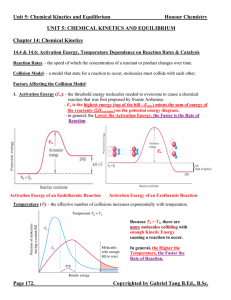
... - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice versa. It appears that the reaction has stopped only because the rate of consumption = rate of production. - if an equilibrium state is disturbed (changing concentrations ...
... - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice versa. It appears that the reaction has stopped only because the rate of consumption = rate of production. - if an equilibrium state is disturbed (changing concentrations ...
Document
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
Chapter
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
Chapter 14 (Kinetics) – Slides and Practice
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
... more surface area for contact with other reactants certain types of chemicals are more reactive than others e.g. potassium metal is more reactive than sodium ...
Rubidium
... Rubidium is not made by the same method as sodium as might have been expected. This is because the rubidium metal, once formed by electrolysis of liquid rubidium chloride (RbCl), is too soluble in the molten salt. cathode: Rb+(l) + e- Rb (l) anode: Cl-(l) 1/2Cl2 (g) + e- ...
... Rubidium is not made by the same method as sodium as might have been expected. This is because the rubidium metal, once formed by electrolysis of liquid rubidium chloride (RbCl), is too soluble in the molten salt. cathode: Rb+(l) + e- Rb (l) anode: Cl-(l) 1/2Cl2 (g) + e- ...
Unit 10 complete 2016-2017
... 1) A camping lantern uses the reaction of calcium carbide and dihydrogen monoxide to produce acetylene gas (C2H2) and calcium hydroxide. You have 1.55 moles of calcium carbide and you need to know how many grams of dihydrogen monoxide to put in the lantern to completely use all the calcium carbide. ...
... 1) A camping lantern uses the reaction of calcium carbide and dihydrogen monoxide to produce acetylene gas (C2H2) and calcium hydroxide. You have 1.55 moles of calcium carbide and you need to know how many grams of dihydrogen monoxide to put in the lantern to completely use all the calcium carbide. ...
Honors Chemistry
... 1) A camping lantern uses the reaction of calcium carbide and dihydrogen monoxide to produce acetylene gas (C2H2) and calcium hydroxide. You have 1.55 moles of calcium carbide and you need to know how many grams of dihydrogen monoxide to put in the lantern to completely use all the calcium carbide. ...
... 1) A camping lantern uses the reaction of calcium carbide and dihydrogen monoxide to produce acetylene gas (C2H2) and calcium hydroxide. You have 1.55 moles of calcium carbide and you need to know how many grams of dihydrogen monoxide to put in the lantern to completely use all the calcium carbide. ...
U6B _13-14
... contains only products -the point where the acid and the base are equal in equal moles ...
... contains only products -the point where the acid and the base are equal in equal moles ...
48th CHEMISTRY OLYMPIAD CHEMISTRY
... These complex compounds A and B can be obtained also at the addition reactions of the corresponding simple substances. 3A + 4H2O → orthosilicic acid + 2C. The substance C is a diprotic acid and it can be decomposed to give complex compounds A and B. Apatite [Ca5 (PO4)3X] contains 3,77 % of an elemen ...
... These complex compounds A and B can be obtained also at the addition reactions of the corresponding simple substances. 3A + 4H2O → orthosilicic acid + 2C. The substance C is a diprotic acid and it can be decomposed to give complex compounds A and B. Apatite [Ca5 (PO4)3X] contains 3,77 % of an elemen ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... homogeneous catalysts for the production of oxygenates [26-33], including methanol, ethanol, and especially ethylene glycol. The principal shortcoming of all homogeneous CO hydrogenation reactions is their low catalytic activity, which results in the need to use high catalyst loadings and drastic re ...
... homogeneous catalysts for the production of oxygenates [26-33], including methanol, ethanol, and especially ethylene glycol. The principal shortcoming of all homogeneous CO hydrogenation reactions is their low catalytic activity, which results in the need to use high catalyst loadings and drastic re ...
guess paper class xii
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
... 15 How many ml of a 0.1M HCl are required to react completely with1 gm mixture of Na 2CO3 and NaHCO3 containing equimolar amounts of two? ...
4U Chemistry Practice Exam - Coristines
... c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increases? a. There is more hydrogen bondin ...
... c. Amines always have a larger molecular weight than amides. d. Amines always have a nitrogen atom attached to two carbon atoms. e. Amines can be found in proteins, but amides can not. 5. Why does the boiling point of an alkane increase as its chain length increases? a. There is more hydrogen bondin ...
Practice Problems in Biomedical Organic Chemistry
... Chemistry: Self-Guided Problems and Answers for Students in Bioorganic and Organic Chemistry, Volume I. (1st Edition) Publisher: Carter, Culver, and Cichewicz, 2016. Retrieved from ...
... Chemistry: Self-Guided Problems and Answers for Students in Bioorganic and Organic Chemistry, Volume I. (1st Edition) Publisher: Carter, Culver, and Cichewicz, 2016. Retrieved from ...
08 Redox Reactions
... In the direct redox reaction, the transferance of electrons is limited to very small distances and therefore, no useful electrical work could be obtained. In these reactions, chemical energy appears as heat. If the transferance of electrons from zinc to copper ions is allowed to occur through some m ...
... In the direct redox reaction, the transferance of electrons is limited to very small distances and therefore, no useful electrical work could be obtained. In these reactions, chemical energy appears as heat. If the transferance of electrons from zinc to copper ions is allowed to occur through some m ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from Fenton’s reaction (1). The most popular electro-Fenton method is the so-called electrogenerated Fenton’s reagent (EFR) [34,37-39], where O2 is bubbled thro ...
... above electrolytic system by adding small amounts of a catalyst like Fe2+, which reacts with electrogenerated H2O2 to yield •OH in solution from Fenton’s reaction (1). The most popular electro-Fenton method is the so-called electrogenerated Fenton’s reagent (EFR) [34,37-39], where O2 is bubbled thro ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.