study material(2014-15) class xii-chemistry
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
Slide 1
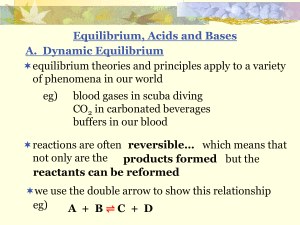
... proposed the law of chemical equilibrium, which states that at a given temperature, a chemical system may reach a state in which a particular ratio of reactant and product concentrations has a constant value. ...
... proposed the law of chemical equilibrium, which states that at a given temperature, a chemical system may reach a state in which a particular ratio of reactant and product concentrations has a constant value. ...
Ch16 - WordPress.com
... The volume of the container was increased at constant temperature and a new equilbrium was established. Predict how each of the following quantities would change at the new equilibrium compared with the initial equilibrium: a concentration of NO2 b mass of NO2 A12. An increase in volume will cause a ...
... The volume of the container was increased at constant temperature and a new equilbrium was established. Predict how each of the following quantities would change at the new equilibrium compared with the initial equilibrium: a concentration of NO2 b mass of NO2 A12. An increase in volume will cause a ...
Study Material - Class- XI- Chemistry
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory *All substances are made up of tiny, indivisible particles called atoms. *Atoms of the same element are identical in shape, size, mass and other properti ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory *All substances are made up of tiny, indivisible particles called atoms. *Atoms of the same element are identical in shape, size, mass and other properti ...
CLUE - virtual laboratories
... commonly presented, both to the public and in college, is both off-putting and ineffective–a potent combination that leads to the widespread public misunderstanding of chemical principles. How many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everythin ...
... commonly presented, both to the public and in college, is both off-putting and ineffective–a potent combination that leads to the widespread public misunderstanding of chemical principles. How many times do we hear about “natural remedies, without drugs or chemicals,” despite the fact that everythin ...
Carefully detach the last page. It is the Data Sheet.
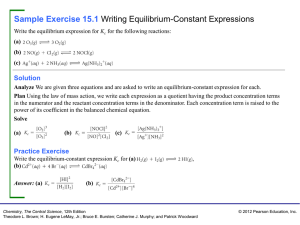
... 18 At a certain temperature, the equilibrium constant for the reaction below is Kp = 0.100. P4(g) U 2 P2(g) In an experiment, some P4 gas was added to an empty reaction vessel and then the vessel was quickly sealed. The total pressure at equilibrium was 1.00 atm. What was the initial pressure of P4 ...
... 18 At a certain temperature, the equilibrium constant for the reaction below is Kp = 0.100. P4(g) U 2 P2(g) In an experiment, some P4 gas was added to an empty reaction vessel and then the vessel was quickly sealed. The total pressure at equilibrium was 1.00 atm. What was the initial pressure of P4 ...
Chapter 3: Stoichiometry
... Decomposition Reactions A single reactant breaks into two or more products ...
... Decomposition Reactions A single reactant breaks into two or more products ...
Question Bank for Pre Board Exam(XII Chemistry)
... 38.Why is Frenkel defect not found in pure alkali metal halides? 39.Which point defect is observed in a crystal when a vacancy is created by an atom missing from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal defect which lowers the de ...
... 38.Why is Frenkel defect not found in pure alkali metal halides? 39.Which point defect is observed in a crystal when a vacancy is created by an atom missing from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal defect which lowers the de ...
Ch16
... The volume of the container was increased at constant temperature and a new equilbrium was established. Predict how each of the following quantities would change at the new equilibrium compared with the initial equilibrium: a concentration of NO2 b mass of NO2 A12. An increase in volume will cause a ...
... The volume of the container was increased at constant temperature and a new equilbrium was established. Predict how each of the following quantities would change at the new equilibrium compared with the initial equilibrium: a concentration of NO2 b mass of NO2 A12. An increase in volume will cause a ...
MC_16_mac
... • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction is absorbed by the w ...
... • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction is absorbed by the w ...
Name:
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.