Drug stability - 성균관대학교 약학대학 물리약학 연구실
... transformed into another molecule which has exactly the same atoms, but the atoms are rearranged * In pharmaceutical aspect, isomerization is the process of conversion of a drug into its optical or geometric isomers, which are often of lower therapeutic activity ...
... transformed into another molecule which has exactly the same atoms, but the atoms are rearranged * In pharmaceutical aspect, isomerization is the process of conversion of a drug into its optical or geometric isomers, which are often of lower therapeutic activity ...
Stoichiometry PP
... The heat of a reaction can be calculated by subtracting the heats of formation of the reactants from the products ...
... The heat of a reaction can be calculated by subtracting the heats of formation of the reactants from the products ...
Nordonia Hills City Schools Honors Chemistry Course of Study
... In a chemical process, recognize that there is an ideal ratio of reactants. 2. Apply ideal ratio concept to reaction coefficients in a balanced equation. 3. Solve stoichiometric problems involving moles and mass. 4. Identify limiting reactant to determine the quantity of product (s) formed. 5. Calcu ...
... In a chemical process, recognize that there is an ideal ratio of reactants. 2. Apply ideal ratio concept to reaction coefficients in a balanced equation. 3. Solve stoichiometric problems involving moles and mass. 4. Identify limiting reactant to determine the quantity of product (s) formed. 5. Calcu ...
17 - Wiley
... structures show that the phenolate anion can distribute its negative charge around the benzene ring, increasing its stability compared with that of the localized O– that results from removal of a proton from ethanol. That is why phenol is a weak acid, whereas alcohols such as ethanol are not acidic. ...
... structures show that the phenolate anion can distribute its negative charge around the benzene ring, increasing its stability compared with that of the localized O– that results from removal of a proton from ethanol. That is why phenol is a weak acid, whereas alcohols such as ethanol are not acidic. ...
A Model For the Calculation of Solvent ... Reaction Rates for Process Design Purposes
... Quantifying the charge distribution evolution during the activation step of a chemical reaction is a difficult task because usually little or no information is known about the structure and charge distribution of the transition state. Since the task at hand is to determine the difference in charge ...
... Quantifying the charge distribution evolution during the activation step of a chemical reaction is a difficult task because usually little or no information is known about the structure and charge distribution of the transition state. Since the task at hand is to determine the difference in charge ...
Calculations from Balanced Equations
... You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reaction. Other reactant ...
... You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reaction. Other reactant ...
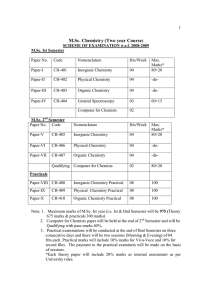
M.Sc. Chemistry (Two year Course)
... each successive quantum level, lowest energy of the particle. Section-B Thermodynamics: Brief resume of first and second Law of thermodynamics. Entropy changes in reversible and irreversible processes; variation of entropy with temperature , pressure and volume, entropy concept as a measure of unava ...
... each successive quantum level, lowest energy of the particle. Section-B Thermodynamics: Brief resume of first and second Law of thermodynamics. Entropy changes in reversible and irreversible processes; variation of entropy with temperature , pressure and volume, entropy concept as a measure of unava ...
class XI CHEMISTRY - Kendriya Vidyalaya No.1 Harni Road
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
... According to this law equal volumes of gases at the same temperature and pressure should contain equal number of molecules. Dalton's Atomic Theory All substances are made up of tiny, indivisible particles called atoms. Atoms of the same element are identical in shape, size, mass and otherproperties. ...
Specification – AS/A Level Chemistry A
... For compounds with giant structures, the term (h) calculate the relative atomic mass of an element given the relative abundances of its relative formula mass will be used. ...
... For compounds with giant structures, the term (h) calculate the relative atomic mass of an element given the relative abundances of its relative formula mass will be used. ...
study material(2014-15) class xii-chemistry
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.