Honors Chemistry: Ch. 12 – Stoichiometry Some useful terms
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
x - mrs. leinweber`s wiki
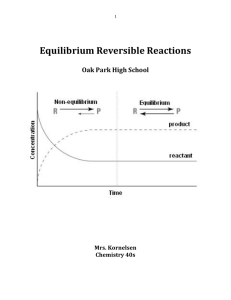
... matter and must have a constant temperature) 4. Equilibrium can be approached from either direction. This means that the equilibrium concentrations will be the same regardless if you started with all reactants, all products, or a mixture of the two ...
... matter and must have a constant temperature) 4. Equilibrium can be approached from either direction. This means that the equilibrium concentrations will be the same regardless if you started with all reactants, all products, or a mixture of the two ...
DCY1B - Manonmaniam Sundaranar University
... is minimum for group VIII elements. This may be due to the repulsion between the added electrons. (v) Ionisation energy: lonisation potential values of most of the d-block elements lie between s and p-block elements. The first ionisation potential values increase as we move across each series. This ...
... is minimum for group VIII elements. This may be due to the repulsion between the added electrons. (v) Ionisation energy: lonisation potential values of most of the d-block elements lie between s and p-block elements. The first ionisation potential values increase as we move across each series. This ...
Exam
... to make 500 mL of a 0.1000 M solution. How many mL of the concentrated solution was needed? 35) A student had 5.0 L of a sulfuric acid solution available, that had a concentration of 1.000 M. The student needed to make 200.0 mL of a 0.2000 M solution. How much of the concentrated solution was needed ...
... to make 500 mL of a 0.1000 M solution. How many mL of the concentrated solution was needed? 35) A student had 5.0 L of a sulfuric acid solution available, that had a concentration of 1.000 M. The student needed to make 200.0 mL of a 0.2000 M solution. How much of the concentrated solution was needed ...
g - Highline Community College
... the surroundings, increasing the entropy of the surroundings When a system process is endothermic, it takes heat from the surroundings, decreasing the entropy of the surroundings The amount the entropy of the surroundings changes depends on its original temperature the higher the original temperat ...
... the surroundings, increasing the entropy of the surroundings When a system process is endothermic, it takes heat from the surroundings, decreasing the entropy of the surroundings The amount the entropy of the surroundings changes depends on its original temperature the higher the original temperat ...
Chapter 4 - AP Chemistry with dr hart
... oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, Aqueous however, most notably in oxyanions. Reactions © 200 ...
... oxidation numbers, although some are positive in certain compounds or ions. Fluorine always has an oxidation number of −1. The other halogens have an oxidation number of −1 when they are negative; they can have positive oxidation numbers, Aqueous however, most notably in oxyanions. Reactions © 200 ...
Chemistry: Percent Yield
... 17: 3.4e Equal volumes of gases at the same temperature and pressure contain an equal number of particles. 33: 3.2b Types of chemical reactions include synthesis, decomposition, single replacement, and double replacement 36: M1.1C – Use algebraic and geometric representations to describe and compare ...
... 17: 3.4e Equal volumes of gases at the same temperature and pressure contain an equal number of particles. 33: 3.2b Types of chemical reactions include synthesis, decomposition, single replacement, and double replacement 36: M1.1C – Use algebraic and geometric representations to describe and compare ...
Packet 1 - Kentucky Community and Technical College System
... How to predict precipitates when solutions of two ionic compounds are mixed Step 1 Write the reactants as they actually exist before any reaction occurs (the complete ionic equation). Remember that when a salt dissolves, its ions completely separate. Step 2 Consider the various solids that coul ...
... How to predict precipitates when solutions of two ionic compounds are mixed Step 1 Write the reactants as they actually exist before any reaction occurs (the complete ionic equation). Remember that when a salt dissolves, its ions completely separate. Step 2 Consider the various solids that coul ...
The integration of flow reactors into synthetic organic chemistry
... would still be easily recognizable to both bench chemists (Figure 1). From a simple analysis of the individual processing steps it is evident that for a single chemical transformation, which may involve only one bond-forming or bond-breaking event, a series of up to six additional manipulations (wor ...
... would still be easily recognizable to both bench chemists (Figure 1). From a simple analysis of the individual processing steps it is evident that for a single chemical transformation, which may involve only one bond-forming or bond-breaking event, a series of up to six additional manipulations (wor ...
+ 2 H2O(l Ca(OH)2 aq)
... c) Sulfur dioxide, SO2, is a nonmetal oxide that reacts with oxygen, O2, to form the higher oxide, SO3. Δ 2 SO (g) 2 SO2(g) + O2(g) ── ...
... c) Sulfur dioxide, SO2, is a nonmetal oxide that reacts with oxygen, O2, to form the higher oxide, SO3. Δ 2 SO (g) 2 SO2(g) + O2(g) ── ...
Chapter 5
... performed with coordinative solvents (methanol or acetone) to help CO dissociation and at 40°C if Ni(CO) 4 is used.13b If Ni(COD) 2 is used instead of Ni(CO) 4 , neither heating nor induction time are required, since the COD ligand is more labile than carbonyl and the oxidative addition takes places ...
... performed with coordinative solvents (methanol or acetone) to help CO dissociation and at 40°C if Ni(CO) 4 is used.13b If Ni(COD) 2 is used instead of Ni(CO) 4 , neither heating nor induction time are required, since the COD ligand is more labile than carbonyl and the oxidative addition takes places ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.