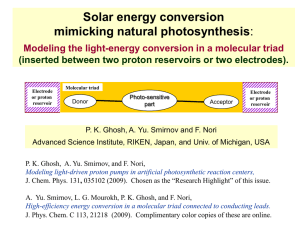
Atomic structure and periodic table
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
... There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table. A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Mosele ...
Heisenberg microscope and which-way experiments
... electron accurately with a very high resolution. Due to Fig. 2.7: Setup of a Heisenberg microscope the wave nature of light, there is a limitation on how close two spots can be and still be seen as two separate spots. This distance is of the order of the wavelenght of light. In Feynman’s analysis of ...
... electron accurately with a very high resolution. Due to Fig. 2.7: Setup of a Heisenberg microscope the wave nature of light, there is a limitation on how close two spots can be and still be seen as two separate spots. This distance is of the order of the wavelenght of light. In Feynman’s analysis of ...
OCR_AS_Level_Chemistry_Unit_F321_Atoms
... Compounds of a metal and a non-metal are made of ions Metal ions have a positive charge Ions of Group 1 elements have a +1 charge, ions of Group 2 elements have a +2 charge For transition elements, like copper and iron, the number after the name gives the charge on the ion e.g. copper(II) oxide cont ...
... Compounds of a metal and a non-metal are made of ions Metal ions have a positive charge Ions of Group 1 elements have a +1 charge, ions of Group 2 elements have a +2 charge For transition elements, like copper and iron, the number after the name gives the charge on the ion e.g. copper(II) oxide cont ...
Carefully detach the last page. It is the Data Sheet.
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
doc: Oxidation Numbers
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
H - Quantum Condensed Matter Research Group
... More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
... More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
Characterization of ultrashort-period GaAsrAlAs superlattices by exciton photoluminescence V.G. Litovchenko
... provides a relaxation mechanism for momentum conservation in xy-plane. This situation is observed for the 8r46 SL. However, for n F 3 an additional effective mechanisms of the indirect zero-phonon recombination is possible. Due to smaller electron effective mass at the X x, y valley Ž0.19m 0 . as co ...
... provides a relaxation mechanism for momentum conservation in xy-plane. This situation is observed for the 8r46 SL. However, for n F 3 an additional effective mechanisms of the indirect zero-phonon recombination is possible. Due to smaller electron effective mass at the X x, y valley Ž0.19m 0 . as co ...
and Lead Bis(tri-tert-butoxystannate)
... density at the germanium or tin atom in compounds such as 1 and 2 not only has a local effect, but affects the whole molecule, and the stereochemical activity of the lone electron pair at the central lead atom is manifested more strongly. In analogy to the definition presented in Ref. [3], the ligan ...
... density at the germanium or tin atom in compounds such as 1 and 2 not only has a local effect, but affects the whole molecule, and the stereochemical activity of the lone electron pair at the central lead atom is manifested more strongly. In analogy to the definition presented in Ref. [3], the ligan ...
Physics Today
... Figure 5. The ground-state configurations of various multielectron atoms, calculated using a Bohr-like model. For both the n = 1 and n = 2 shells, the electron locations (red dots) found from that simplistic model agree closely with the maximum charge densities obtained from standard quantum-theory ...
... Figure 5. The ground-state configurations of various multielectron atoms, calculated using a Bohr-like model. For both the n = 1 and n = 2 shells, the electron locations (red dots) found from that simplistic model agree closely with the maximum charge densities obtained from standard quantum-theory ...
Physics Today
... two beams f(z,t) and g(z,i) are the same at z = 0, and in Physical Research Laboratory in Navrangpura, India, has which initially all of the atoms are in the ground state. (The shown the matching of photon statistics.7 pulses need not have equal Rabi frequencies Op and flc.) For EIT to work, it must ...
... two beams f(z,t) and g(z,i) are the same at z = 0, and in Physical Research Laboratory in Navrangpura, India, has which initially all of the atoms are in the ground state. (The shown the matching of photon statistics.7 pulses need not have equal Rabi frequencies Op and flc.) For EIT to work, it must ...
1AMQ, Part II Quantum Mechanics
... Light emitted by free atoms has fixed or discrete wavelengths. Only certain energies of photons can occur (unlike the continuous spectrum observed from a Black Body). Atoms can absorb energy (become excited) by collisions, fluorescence (absorption and reemission of light) etc. The emitted light can ...
... Light emitted by free atoms has fixed or discrete wavelengths. Only certain energies of photons can occur (unlike the continuous spectrum observed from a Black Body). Atoms can absorb energy (become excited) by collisions, fluorescence (absorption and reemission of light) etc. The emitted light can ...
“Midterm” Exam # 1 - Elgin Community College
... make up a number and use that for the rest of the problem. Best of luck! 14.7 psi = 1 atm = 760 mm Hg, Avogadro’s number 6.02x1023, R = 0.082 L*atm*K-1*mol-1, R = 8.314 J*K-1*mol-1 1) (4 pts) Name the following substances: NaHCO3 Sodium bicarbonate ...
... make up a number and use that for the rest of the problem. Best of luck! 14.7 psi = 1 atm = 760 mm Hg, Avogadro’s number 6.02x1023, R = 0.082 L*atm*K-1*mol-1, R = 8.314 J*K-1*mol-1 1) (4 pts) Name the following substances: NaHCO3 Sodium bicarbonate ...
Quantum State Control via Trap-induced Shape Resonance in
... gates can be performed within a well-defined “logical basis”. This constraint can be overcome by placing the particles in distinguishable locations where the atomic quantum numbers are conserved asymptotically. Under typical conditions such separated atoms would generally encounter very weak interac ...
... gates can be performed within a well-defined “logical basis”. This constraint can be overcome by placing the particles in distinguishable locations where the atomic quantum numbers are conserved asymptotically. Under typical conditions such separated atoms would generally encounter very weak interac ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.