Conceptual Integrated Science The Elements The Periodic Table
... Bohr reasoned that there must be a number of distinct energy levels within the atom. Each energy level has a principal quantum number n, where n is always an integer. The lowest level is n = 1 and is closest to the nucleus. Electrons release energy in discrete amounts that form discrete lines in the ...
... Bohr reasoned that there must be a number of distinct energy levels within the atom. Each energy level has a principal quantum number n, where n is always an integer. The lowest level is n = 1 and is closest to the nucleus. Electrons release energy in discrete amounts that form discrete lines in the ...
File
... This occurs if each of the atoms have the same pull on the electron pair in the bond (equal electronegativity). It can be found in molecules of elements such as O2, Br2 and N2. Since the atoms in each of these molecules are the same they will have the same electronegativity. ...
... This occurs if each of the atoms have the same pull on the electron pair in the bond (equal electronegativity). It can be found in molecules of elements such as O2, Br2 and N2. Since the atoms in each of these molecules are the same they will have the same electronegativity. ...
Close-coupling study of rotational energy transfer of CO(v=2) by
... RET for CO(v = 2) by Bodo et al. [13] for j = 1 between 10−5 and 1 cm−1 . For this collision energy range, the only available RET channel is deexcitation to v 0 = 2, j 0 = 0 for which we find very good agreement. ...
... RET for CO(v = 2) by Bodo et al. [13] for j = 1 between 10−5 and 1 cm−1 . For this collision energy range, the only available RET channel is deexcitation to v 0 = 2, j 0 = 0 for which we find very good agreement. ...
Chapter 2 - San Joaquin Memorial High School
... The Greeks were the first to try to explain why chemical changes occur. By about 400 B.C. they had proposed that all matter was composed of four fundamental substances: fire, earth, water, and air. The Greeks also considered the question of whether matter is continuous, and thus infinitely divisible ...
... The Greeks were the first to try to explain why chemical changes occur. By about 400 B.C. they had proposed that all matter was composed of four fundamental substances: fire, earth, water, and air. The Greeks also considered the question of whether matter is continuous, and thus infinitely divisible ...
Imaging resonances in low-energy NO-He inelastic collisions REPORTS
... of the molecule. Using cryogenically cooled molecular species such as CO and O2, partial-wave resonances were observed in the state-to-state ICSs for inelastic collisions with He and H2 target beams at energies down to 4 K (14–16). The measurement of DCSs at scattering resonances remains a largely u ...
... of the molecule. Using cryogenically cooled molecular species such as CO and O2, partial-wave resonances were observed in the state-to-state ICSs for inelastic collisions with He and H2 target beams at energies down to 4 K (14–16). The measurement of DCSs at scattering resonances remains a largely u ...
Chemistry English
... Each electron in an atom possesses a total energy (kinetic plus potential). The lowest-energy electrons are those closest to the nucleus of the atom and the most difficult to remove from the atom. Niels Bohr (1885-1962), a Danish physicist, first introduced the idea of electronic n: principal quantu ...
... Each electron in an atom possesses a total energy (kinetic plus potential). The lowest-energy electrons are those closest to the nucleus of the atom and the most difficult to remove from the atom. Niels Bohr (1885-1962), a Danish physicist, first introduced the idea of electronic n: principal quantu ...
Adiabatic creation of coherent superposition states in atomic beams
... One of the techniques frequently used for robust population transfer, and potentially available for the creation of superpositions, is stimulated Raman adiabatic passage (STIRAP) [6]. This technique uses two pulses, offset from each other in time, and the Raman coupling !a ↔ !c ↔ !b. One of the cond ...
... One of the techniques frequently used for robust population transfer, and potentially available for the creation of superpositions, is stimulated Raman adiabatic passage (STIRAP) [6]. This technique uses two pulses, offset from each other in time, and the Raman coupling !a ↔ !c ↔ !b. One of the cond ...
Full Text PDF
... the second group μeffisbgerthanμSo,.p-biculngrsoyfthe ions of the second group. This trend can be easily justified if one supposes that kSE increases as the density of unpaired metal electrons increases at the collision site between complex and Ps atom (i.e. at the _complex boundaries and that the e ...
... the second group μeffisbgerthanμSo,.p-biculngrsoyfthe ions of the second group. This trend can be easily justified if one supposes that kSE increases as the density of unpaired metal electrons increases at the collision site between complex and Ps atom (i.e. at the _complex boundaries and that the e ...
Source
... the context of the Simple Line Access Protocol defined by the IVOA (c.f. Ref[] IVOA Simple Line Access protocol) The main objective of the model is to integrate with and support the Simple Line Access Protocol, with which it forms a compact unit. This integration allows seamless access to Spectral L ...
... the context of the Simple Line Access Protocol defined by the IVOA (c.f. Ref[] IVOA Simple Line Access protocol) The main objective of the model is to integrate with and support the Simple Line Access Protocol, with which it forms a compact unit. This integration allows seamless access to Spectral L ...
Line junctions in the quantum Hall effect - Penn Physics
... j (x);e i d kx where d k is a gauge-invariant momentum difference between the right and left moving modes. If the edge modes are separated by a distance d then d k52 p Bd/F 0 , where B is the applied magnetic field, and F 0 5hc/e is the magnetic flux quantum. However, in any real device one expects ...
... j (x);e i d kx where d k is a gauge-invariant momentum difference between the right and left moving modes. If the edge modes are separated by a distance d then d k52 p Bd/F 0 , where B is the applied magnetic field, and F 0 5hc/e is the magnetic flux quantum. However, in any real device one expects ...
Spatial ordering of charge and spin in quasi-one
... for one (even or odd) spatial parity of the state strictly dependent on the number of electrons. We present this dependence in the form of a theorem for which we provide a rigorous analytical proof. The found dependence of the parity of one-dimensional Wigner molecule states on the number of electro ...
... for one (even or odd) spatial parity of the state strictly dependent on the number of electrons. We present this dependence in the form of a theorem for which we provide a rigorous analytical proof. The found dependence of the parity of one-dimensional Wigner molecule states on the number of electro ...
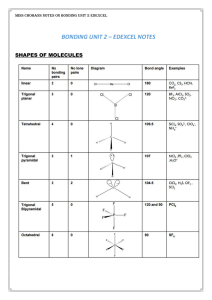
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.