SCH 4U REVIEW Notes
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
FREE Sample Here
... 47) When the atoms involved in a covalent bond have the same electronegativity, what type of bond results? A) an ionic bond B) a hydrogen bond C) a nonpolar covalent bond D) a polar covalent bond Answer: C Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 48) Nitrogen (N) normally forms three c ...
... 47) When the atoms involved in a covalent bond have the same electronegativity, what type of bond results? A) an ionic bond B) a hydrogen bond C) a nonpolar covalent bond D) a polar covalent bond Answer: C Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 48) Nitrogen (N) normally forms three c ...
CHAPTER 23 The Interaction of Light with Matter: I
... strength of this Comptonization is measure for the electron pressure Pe ∝ ne Te along the line-of-sight through the cluster. Observations of the SZ effect provide a nearly redshift-independent means of detecting galaxy clusters. Resonant scattering: Resonant scattering, also known as line scattering ...
... strength of this Comptonization is measure for the electron pressure Pe ∝ ne Te along the line-of-sight through the cluster. Observations of the SZ effect provide a nearly redshift-independent means of detecting galaxy clusters. Resonant scattering: Resonant scattering, also known as line scattering ...
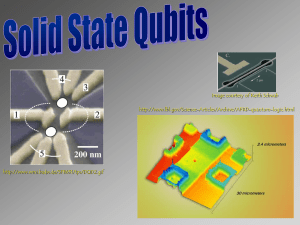
superconducting qubits solid state qubits
... “Charge qubits” and “spin qubits” The qubits levels can be formed by either the energy levels of an electron in a potential well (such as a quantum dot or an impurity ion) or by the spin states of the electron (or the nucleus). The former are examples of charge qubits. The charge qubits have high e ...
... “Charge qubits” and “spin qubits” The qubits levels can be formed by either the energy levels of an electron in a potential well (such as a quantum dot or an impurity ion) or by the spin states of the electron (or the nucleus). The former are examples of charge qubits. The charge qubits have high e ...
Phase switching in a voltage-biased Aharonov-Bohm interferometer Vadim I. Puller
... A possible explanation for this effect may be as the result of electrostatic AB oscillations, as was studied in Refs. 11 and 12. Some features of the experiment, however, are inconsistent with such an explanation. In particular, as mentioned above, there is apparent correlation between the onset of ...
... A possible explanation for this effect may be as the result of electrostatic AB oscillations, as was studied in Refs. 11 and 12. Some features of the experiment, however, are inconsistent with such an explanation. In particular, as mentioned above, there is apparent correlation between the onset of ...
sch4ureview
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
12 U Chem Review
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
... organic compound – a compound that contains carbon and usually hydrogen catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical character ...
FREE Sample Here
... 47) When the atoms involved in a covalent bond have the same electronegativity, what type of bond results? A) an ionic bond B) a hydrogen bond C) a nonpolar covalent bond D) a polar covalent bond Answer: C Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 48) Nitrogen (N) normally forms three c ...
... 47) When the atoms involved in a covalent bond have the same electronegativity, what type of bond results? A) an ionic bond B) a hydrogen bond C) a nonpolar covalent bond D) a polar covalent bond Answer: C Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 48) Nitrogen (N) normally forms three c ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Document
... unit cell, without including thermal energy and entropy, therefore at T = 0 K. The functional adopted was B3LYP25 and the basis set was Pople-type 6-31G(2d,2p), which is one of the largest basis set that can be used in these kinds of calculations. Optical properties of the periodic system were calcu ...
... unit cell, without including thermal energy and entropy, therefore at T = 0 K. The functional adopted was B3LYP25 and the basis set was Pople-type 6-31G(2d,2p), which is one of the largest basis set that can be used in these kinds of calculations. Optical properties of the periodic system were calcu ...
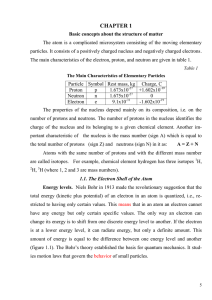
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.