CHM1045 General Chemistry and Qualitative Analysis
... Relating the type of bond with the electronegativity differences of the elements involved in bonding. Comparing and contrasting the differences between ionic and covalent bonding. Writing the Lewis electron dot structure of elements, ions, ionic compounds, and covalent compounds. Recognizing excepti ...
... Relating the type of bond with the electronegativity differences of the elements involved in bonding. Comparing and contrasting the differences between ionic and covalent bonding. Writing the Lewis electron dot structure of elements, ions, ionic compounds, and covalent compounds. Recognizing excepti ...
QUANTUM-MECHANICAL MODEL OF THE ATOM Quantum
... Angular momentum quantum number (l): an integer from 0 to n-1 Indicates the shape of the orbital, sometimes called as “orbital-shape quantum number” n limits l Example: for orbital with n=1 ==> l=0; n=2 ==> l=0,1 Magnetic quantum number (ml): an integer from -1 through 0 to +1 prescribes ...
... Angular momentum quantum number (l): an integer from 0 to n-1 Indicates the shape of the orbital, sometimes called as “orbital-shape quantum number” n limits l Example: for orbital with n=1 ==> l=0; n=2 ==> l=0,1 Magnetic quantum number (ml): an integer from -1 through 0 to +1 prescribes ...
Chp 1,2 rev
... 3) If a sample starts at 200g how much will be left after 5 half-life’s have gone by? ...
... 3) If a sample starts at 200g how much will be left after 5 half-life’s have gone by? ...
General Chemistry: An Integrated Approach
... • When an electron has been promoted to a higher level, the electron (and the atom) is in an excited state. • Electrons are promoted to higher levels through an electric discharge, heat, or some other source of energy. • An atom in an excited state eventually emits a photon (or several) as the elect ...
... • When an electron has been promoted to a higher level, the electron (and the atom) is in an excited state. • Electrons are promoted to higher levels through an electric discharge, heat, or some other source of energy. • An atom in an excited state eventually emits a photon (or several) as the elect ...
Vocabulary:
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
... Bohr’s Atomic Model Planetary System Model – Electrons move around the nucleus of an atom, like the planets around the sun. James Maxwell – Proposed that visible light consists of electromagnetic waves. Maxwell Planck – Suggested that atoms and molecules emit energy in discrete quantities, called qu ...
Nondispersing Bohr Wave Packets - Physics (APS)
... HCP relative to the MW field is slowly scanned over many laser shots. If the HCP arrives before the MW pulse (1), we see no variation in the signal as the delay of the HCP is scanned for either polarization, as expected; the atoms are in the 72p state, an eigenstate. If the HCP arrives at (2), when ...
... HCP relative to the MW field is slowly scanned over many laser shots. If the HCP arrives before the MW pulse (1), we see no variation in the signal as the delay of the HCP is scanned for either polarization, as expected; the atoms are in the 72p state, an eigenstate. If the HCP arrives at (2), when ...
Chapter 5
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
smart_materials_1 - Aldercar High School
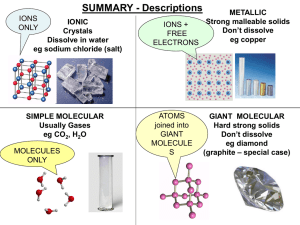
... CONDUCT: YES (very well) Free electrons between ions ...
... CONDUCT: YES (very well) Free electrons between ions ...
So where did all the matter on Earth come from - Bennatti
... from chemical reactions. They are called nuclear reactions because they involve changes in the nuclei of atoms. These changes in the nuclei of the atoms cause the atoms to change from one element to another as the number of protons changes. We will talk about this a bit more later. ...
... from chemical reactions. They are called nuclear reactions because they involve changes in the nuclei of atoms. These changes in the nuclei of the atoms cause the atoms to change from one element to another as the number of protons changes. We will talk about this a bit more later. ...
Teacher quality grant - Gulf Coast State College
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
Teacher quality grant
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
... An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron ...
Modern Physics 342
... Energy Levels and Spectroscopic Notation The notation for the quantum state of an electron is now described by the four quantum numbers n, l, ml and ms . For example, the ground state of the hydrogen is labeled as (n,l,ml,ms )=(1,0,0,±½) which means the are two quantum states that can be occupied b ...
... Energy Levels and Spectroscopic Notation The notation for the quantum state of an electron is now described by the four quantum numbers n, l, ml and ms . For example, the ground state of the hydrogen is labeled as (n,l,ml,ms )=(1,0,0,±½) which means the are two quantum states that can be occupied b ...
Atomic Structure
... rest, at times, it behaves as if it has mass. Einstein’s equation was confirmed by experiments done by Arthur Compton in 1922. Collisions between X-rays and electrons confirmed the “mass” of the radiation. ...
... rest, at times, it behaves as if it has mass. Einstein’s equation was confirmed by experiments done by Arthur Compton in 1922. Collisions between X-rays and electrons confirmed the “mass” of the radiation. ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.