AP Chemistry - Freehold Regional High School District
... a depth of understanding of fundamentals and a reasonable competence in dealing with chemical problems. The course should contribute to the development of the students’ abilities to think clearly and to express their ideas, orally and in writing with clarity and logic. The college course in general ...
... a depth of understanding of fundamentals and a reasonable competence in dealing with chemical problems. The course should contribute to the development of the students’ abilities to think clearly and to express their ideas, orally and in writing with clarity and logic. The college course in general ...
Chapter 16.1
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
Aggregation and Adsorption at Interfaces
... migration of the surfactant to the surface is a spontaneous process. At the gasliquid interface, the result is the creation of new unit area of surface and the formation of an oriented surfactant monolayer with the hydrophobic tails pointing out of, and the head group inside, the water phase. The ba ...
... migration of the surfactant to the surface is a spontaneous process. At the gasliquid interface, the result is the creation of new unit area of surface and the formation of an oriented surfactant monolayer with the hydrophobic tails pointing out of, and the head group inside, the water phase. The ba ...
Chapter 16 Aqueous Ionic Equilibrium Lecture Presentation
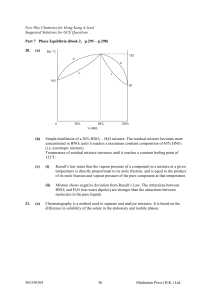
... • It is a plot of pH versus the amount of added titrant. • The inflection point of the curve is the equivalence point of the titration. • Prior to the equivalence point, the known solution in the flask is in excess, so the pH is closest to its pH. • The pH of the equivalence point depends on the pH ...
... • It is a plot of pH versus the amount of added titrant. • The inflection point of the curve is the equivalence point of the titration. • Prior to the equivalence point, the known solution in the flask is in excess, so the pH is closest to its pH. • The pH of the equivalence point depends on the pH ...
Bis2A 06.Appendix A review of Red/Ox reactions
... Humans interact with one another in various and complex ways, and we classify these interactions according to common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their sts or feet, we say they are ghting. Faced with a wide ...
... Humans interact with one another in various and complex ways, and we classify these interactions according to common patterns of behavior. When two humans exchange information, we say they are communicating. When they exchange blows with their sts or feet, we say they are ghting. Faced with a wide ...
Title Pressure effect on the eda
... studied that the spectral shift in the absorption spectra of the EDA-complexes in several solvents. They showed that the polar solvents otten cau=_eda blue shift relative to non-polar solvents and that this effect could not be described by the classical soh-ation theory. They discussed the blue shin ...
... studied that the spectral shift in the absorption spectra of the EDA-complexes in several solvents. They showed that the polar solvents otten cau=_eda blue shift relative to non-polar solvents and that this effect could not be described by the classical soh-ation theory. They discussed the blue shin ...
(a) From , 2013 General Chemistry I
... states that the change in internal energy (DU) is the sum of the work and heat changes: it is applicable to any process that begins and ends in equilibrium states. ...
... states that the change in internal energy (DU) is the sum of the work and heat changes: it is applicable to any process that begins and ends in equilibrium states. ...
Effect of nature and surface density of oxygen species on product
... uncertainty in the steady-state distribution of the above forms of active sites, i.e. [O]S, [OH]S and [ ]S. In real experiments such distribution is reached after some transition period, during which the total amount of reactants has passed through the catalyst layer, can by far exceed the number of ...
... uncertainty in the steady-state distribution of the above forms of active sites, i.e. [O]S, [OH]S and [ ]S. In real experiments such distribution is reached after some transition period, during which the total amount of reactants has passed through the catalyst layer, can by far exceed the number of ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.