atoms - Chemistry 7
... charged particles that we now know are electrons. He determines the charge/mass ratio of a single electron to be 1.76 x 108 C/g. • Cathode Rays are deflected by magnetic ...
... charged particles that we now know are electrons. He determines the charge/mass ratio of a single electron to be 1.76 x 108 C/g. • Cathode Rays are deflected by magnetic ...
Chapter 2 Expanded Notes
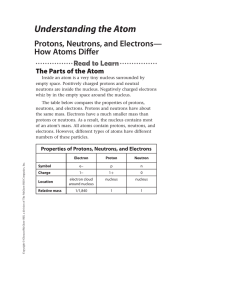
... number of them can be altered. There are several subtle things to note about the table: 1. There is only positive charge, the proton, and negative charge, the electron. Neutrons are neutral, they have no charge. 2. The charges between the protons and electrons are perfectly matched at 1. We say they ...
... number of them can be altered. There are several subtle things to note about the table: 1. There is only positive charge, the proton, and negative charge, the electron. Neutrons are neutral, they have no charge. 2. The charges between the protons and electrons are perfectly matched at 1. We say they ...
Identify the letter of the choice that best completes
... 2) Rutherford conducted an investigation where particles were fired at gold atoms. Some of the particles went through the gold and some bounced directly back or at angles. What did this observation confirm about the structure of an atom? a. Atoms are mostly empty space. b. Atoms are totally solid an ...
... 2) Rutherford conducted an investigation where particles were fired at gold atoms. Some of the particles went through the gold and some bounced directly back or at angles. What did this observation confirm about the structure of an atom? a. Atoms are mostly empty space. b. Atoms are totally solid an ...
Atomic Structure
... • Law of Definite Composition ─ a compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound • Example: teaspoon and a cup of sugar both are composed of 42.1 % ...
... • Law of Definite Composition ─ a compound contains the same elements in exactly the same proportions by mass regardless of the size of the sample or source of the compound • Example: teaspoon and a cup of sugar both are composed of 42.1 % ...
Atoms PPT
... mixture of all an atoms isotopes. The average atomic mass is close to the mass of its most abundant isotope. • This is the number found on the periodic table ...
... mixture of all an atoms isotopes. The average atomic mass is close to the mass of its most abundant isotope. • This is the number found on the periodic table ...
document
... Atoms of the same element with different numbers of neutrons http://www.sci.tamucc.edu/pals/morvant/genchem/atomic/page9.htm Nuclide – general term for any isotope of any element Each isotope has a % abundance in nature Symbols for isotopes: Lithium – 6 / Lithium – 7 Isotopes differ by Number of ...
... Atoms of the same element with different numbers of neutrons http://www.sci.tamucc.edu/pals/morvant/genchem/atomic/page9.htm Nuclide – general term for any isotope of any element Each isotope has a % abundance in nature Symbols for isotopes: Lithium – 6 / Lithium – 7 Isotopes differ by Number of ...
UNIT 1 Atomic Structure
... Note electrons are usually shown as far apart as possible -they have the same charge and therefore repel each other. 3. The Noble gases (Group 0) have a stable electron configuration (s2p6) with 8 electrons filling the outer s and p orbitals. This stability comes from the low energy state of this c ...
... Note electrons are usually shown as far apart as possible -they have the same charge and therefore repel each other. 3. The Noble gases (Group 0) have a stable electron configuration (s2p6) with 8 electrons filling the outer s and p orbitals. This stability comes from the low energy state of this c ...
Understanding the Atom
... mass number = number of protons + number of neutrons You can determine any one of these three quantities if you know the value of the other two quantities. For example, to determine the mass number of an atom, you must know the number of neutrons and the number of protons in the atom. The mass numbe ...
... mass number = number of protons + number of neutrons You can determine any one of these three quantities if you know the value of the other two quantities. For example, to determine the mass number of an atom, you must know the number of neutrons and the number of protons in the atom. The mass numbe ...
1 - Hobbs Freshman High School
... 11. In an experiment Rutherford bombarded gold foil with high-speed positively charges particles and found, that while most of the particles passed through this foil, a few bounced back. In terms of atomic structure, which of the following statements explains this observation? (The atom is mostly em ...
... 11. In an experiment Rutherford bombarded gold foil with high-speed positively charges particles and found, that while most of the particles passed through this foil, a few bounced back. In terms of atomic structure, which of the following statements explains this observation? (The atom is mostly em ...
Chem Test 2 - TeacherWeb
... b. weighted average of the masses of the isotopes of the element c. total mass of the isotopes of the element d. average of the mass number and the atomic number for the element The atomic mass of an element depends upon the ____. a. mass of each electron in that element b. mass of each isotope of t ...
... b. weighted average of the masses of the isotopes of the element c. total mass of the isotopes of the element d. average of the mass number and the atomic number for the element The atomic mass of an element depends upon the ____. a. mass of each electron in that element b. mass of each isotope of t ...
How_to_draw_a_(Bohr) - Mrs. GM Biology 200
... numbers) & round to the nearest whole number… So, for Sodium (Na), the atomic mass is 22.99, but rounded to the nearest whole number it’s 23. • atomic mass = # of protons + # of neutrons ...
... numbers) & round to the nearest whole number… So, for Sodium (Na), the atomic mass is 22.99, but rounded to the nearest whole number it’s 23. • atomic mass = # of protons + # of neutrons ...
Summary of Chapter 2
... • You need to know the ions of which it is composed. • The formula must reflect the electrical neutrality of the compound. • You must combine cations and anions in a ratio so that the total positive charge is equal to the total negative charge. • Example: Consider the formation of Mg3N2: • Mg loses ...
... • You need to know the ions of which it is composed. • The formula must reflect the electrical neutrality of the compound. • You must combine cations and anions in a ratio so that the total positive charge is equal to the total negative charge. • Example: Consider the formation of Mg3N2: • Mg loses ...
Atomic Structure
... system Electrons revolve (orbit) around the nucleus Principle energy levels (PEL) = rings around the nucleus ...
... system Electrons revolve (orbit) around the nucleus Principle energy levels (PEL) = rings around the nucleus ...
Chapter 2 PPT - Richsingiser.com
... • Often the same elements form more than one compound. Numerical prefixes are used to give the number of atoms present in the molecule. Number one two three four five six ...
... • Often the same elements form more than one compound. Numerical prefixes are used to give the number of atoms present in the molecule. Number one two three four five six ...
Atoms/Atomic Theory PPT
... masses of atoms are so small, it is more convenient to use relative atomic masses instead of real masses to set up a scale, we have to pick one atom to be the standard since 1961, the carbon-12 nuclide is the standard and is assigned a mass of ...
... masses of atoms are so small, it is more convenient to use relative atomic masses instead of real masses to set up a scale, we have to pick one atom to be the standard since 1961, the carbon-12 nuclide is the standard and is assigned a mass of ...
Discovery of Atomic Structure
... Electrons are each ~ 9.11 x 10-28 g. Use atomic mass unit (amu) instead of gram. The mass of one proton is ~ 1 amu. ...
... Electrons are each ~ 9.11 x 10-28 g. Use atomic mass unit (amu) instead of gram. The mass of one proton is ~ 1 amu. ...
atoms
... Atoms of any one element are different from those of any other element. 3) Atoms of different elements combine in simple whole-number ratios to form chemical compounds. E.g. CO2 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
... Atoms of any one element are different from those of any other element. 3) Atoms of different elements combine in simple whole-number ratios to form chemical compounds. E.g. CO2 4) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. ...
Atomic Theory
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
Welcome to Chemistry 1001
... • Atomic Number = number of protons • # of protons determines kind of atom • The same as the number of electrons in the neutral atom • Mass Number = the number of protons ...
... • Atomic Number = number of protons • # of protons determines kind of atom • The same as the number of electrons in the neutral atom • Mass Number = the number of protons ...