Chemistry
... 2000 years later Dalton proposed atomic theory (performed experimental science) Summarize Dalton’s atomic theory states: Elements made up of submicroscopic indivisible particles called atoms Atoms of same element identical Atoms of different elements are different Atoms of different elements can phy ...
... 2000 years later Dalton proposed atomic theory (performed experimental science) Summarize Dalton’s atomic theory states: Elements made up of submicroscopic indivisible particles called atoms Atoms of same element identical Atoms of different elements are different Atoms of different elements can phy ...
end of year review
... _____ 5. One way that mixtures differ from pure substances is in the methods that can be used to separate them into their components. Which of the following is a method used to separate the components of some mixtures? A. a nuclear reaction C. a chemical reaction B. a filtration process ...
... _____ 5. One way that mixtures differ from pure substances is in the methods that can be used to separate them into their components. Which of the following is a method used to separate the components of some mixtures? A. a nuclear reaction C. a chemical reaction B. a filtration process ...
Period:______ Table Number
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ____________________ of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher __________________________ ga ...
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ____________________ of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher __________________________ ga ...
`atoms`. - MrsCoxsChemistryCorner
... • Many people respected his ideas, therefore the atomic theory that was proposed 100 years earlier was rejected for the next 2000 years. ...
... • Many people respected his ideas, therefore the atomic theory that was proposed 100 years earlier was rejected for the next 2000 years. ...
Chemistry You Need to Know
... He couldn’t find elements to fit all the property trends, so he left holes ...
... He couldn’t find elements to fit all the property trends, so he left holes ...
Aleksander Herman
... Our approach differs from the common method in that, the optimal exchange parameter αex was estimated for each atom in the periodic table [11,12]. Originally, this idea was introduced by Slater and Johnson [13]. In section IV of their paper, they have suggested a criterion for determining a value of ...
... Our approach differs from the common method in that, the optimal exchange parameter αex was estimated for each atom in the periodic table [11,12]. Originally, this idea was introduced by Slater and Johnson [13]. In section IV of their paper, they have suggested a criterion for determining a value of ...
Document
... • Each element has a definite and fixed number of protons. If the number of protons changes, then the atom becomes a different element. • Changes in the number of particles in the nucleus (protons or neutrons) is very rare. It only takes place in nuclear processes such as: ...
... • Each element has a definite and fixed number of protons. If the number of protons changes, then the atom becomes a different element. • Changes in the number of particles in the nucleus (protons or neutrons) is very rare. It only takes place in nuclear processes such as: ...
Electrons - TeacherWeb
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
Electrons - Chemistry Geek
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
Chapter 18 - Powell County Schools
... Atomic mass, mass number, and isotopes Mass number In addition to the atomic number, every atomic nucleus can be described by its mass number. The mass number is equal to the total number of protons plus neutrons in the nucleus of an atom. Recall that atoms of the same element have the same number o ...
... Atomic mass, mass number, and isotopes Mass number In addition to the atomic number, every atomic nucleus can be described by its mass number. The mass number is equal to the total number of protons plus neutrons in the nucleus of an atom. Recall that atoms of the same element have the same number o ...
Notes 4.3 filled in
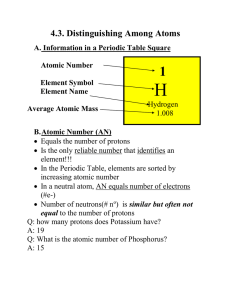
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
... B. Atomic Number (AN) Equals the number of protons Is the only reliable number that identifies an element!!! In the Periodic Table, elements are sorted by increasing atomic number In a neutral atom, AN equals number of electrons (#e-) Number of neutrons(# no) is similar but often not equal ...
atomic structure studyguide key
... b.Which of his points was later proven incorrect? Atoms are indivisible: Later proven they are composed of subatomic particles. Atoms of the same element are the same: Later discovered isotopes of elements and they are different in the number of neutrons and mass number. c. Illustrate his model ...
... b.Which of his points was later proven incorrect? Atoms are indivisible: Later proven they are composed of subatomic particles. Atoms of the same element are the same: Later discovered isotopes of elements and they are different in the number of neutrons and mass number. c. Illustrate his model ...
Electrons - Irion County ISD
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
... A list of all the electrons in an atom (or ion) • Must go in order (Aufbau principle) • 2 electrons per orbital, maximum • We need electron configurations so that we can determine the number of electrons in the outermost energy level. These are called valence electrons. • The number of valence elect ...
Chemistry I Syllabus 2011-2012
... Weeks 5—10: Chapter 2 Fun with the Periodic Table, Active Chemistry Pages: 96 – 192 Essential Questions: 1. What specific properties of materials allow them to be classified as metals or nonmetals? 2. How is the relative mass of atoms determined? What does that indicate about the way in which they ...
... Weeks 5—10: Chapter 2 Fun with the Periodic Table, Active Chemistry Pages: 96 – 192 Essential Questions: 1. What specific properties of materials allow them to be classified as metals or nonmetals? 2. How is the relative mass of atoms determined? What does that indicate about the way in which they ...
Atomic Number
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
Study Guide Answer Key
... 2. Consider an element Z that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 19.0 is 55.0% abundant; the isotope with a mass number of 21.0 is 45.0% abundant. What is the average atomic mass for element Z? [(mass A) (%A)] + [(mass B) (%B ...
... 2. Consider an element Z that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 19.0 is 55.0% abundant; the isotope with a mass number of 21.0 is 45.0% abundant. What is the average atomic mass for element Z? [(mass A) (%A)] + [(mass B) (%B ...
Chemistry Atoms Learning Objectives Atoms Essential knowledge
... The atomic number of an element is the same as the number of protons. In a neutral atom, the number of electrons is the same as the number of protons. All atoms of an element have the same number of protons. The average atomic mass for each element is the weighted average of that element’s naturally ...
... The atomic number of an element is the same as the number of protons. In a neutral atom, the number of electrons is the same as the number of protons. All atoms of an element have the same number of protons. The average atomic mass for each element is the weighted average of that element’s naturally ...
Atomic Structures Part
... Each element differs from the preceding element by having one more positive charge in its nucleus ...
... Each element differs from the preceding element by having one more positive charge in its nucleus ...
Unit 1: Basic Chemistry for Biology QUIZ STUDY GUIDE Things to
... -Know the difference between a covalent bond and an ionic bond. -Be able to answer questions about a bond similar to the ones on ...
... -Know the difference between a covalent bond and an ionic bond. -Be able to answer questions about a bond similar to the ones on ...