Chapter 2
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
... in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. Isotopes of an element differ in the number of neutrons, and thus in mass ...
Symbols of Elements
... • is a three-dimensional space around a nucleus, where an electron is most likely to be found. ...
... • is a three-dimensional space around a nucleus, where an electron is most likely to be found. ...
Atoms The configuration of subatomic particles within an
... 7. A sample of a gas to be used in a cathode ray tube has a mass of 0.04161 grams and a volume of 3.8 cubic centimeters. To which number of significant figures should the calculated density of the sample be expressed? ...
... 7. A sample of a gas to be used in a cathode ray tube has a mass of 0.04161 grams and a volume of 3.8 cubic centimeters. To which number of significant figures should the calculated density of the sample be expressed? ...
binary molecular compounds
... • The algebraic sum of the oxidation numbers is always equal to 0, as long as the compound is neutral • The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion • Oxidation numbers can also be assigned to ions • monoatomic ions have oxidation numb ...
... • The algebraic sum of the oxidation numbers is always equal to 0, as long as the compound is neutral • The algebraic sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion • Oxidation numbers can also be assigned to ions • monoatomic ions have oxidation numb ...
Atomic Theory
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
Atoms - Issaquah Connect
... • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Notes Unit 3
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
Nontes Unit 3 pdf
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
... Neutron stars can be formed when stars use up all of their fuel. Protons and electrons in the star merge to form neutrons and neutrinos. The neutrons form the neutron star, which is usually around 20 km in diameter, but can be over twice the mass of the sun. Nuclear fission reactions occur when a fr ...
All matter is made up of tiny particles called atoms
... across the 50-yard line. In spite of this size difference, virtually an of the mass of an atom is concentrated in its nucleus. One electron, which has a negative charge, weighs only 1/1836 as much as the lightest of all nuclei, that of the hydrogen atom (proton). In addition, all the particles (pro ...
... across the 50-yard line. In spite of this size difference, virtually an of the mass of an atom is concentrated in its nucleus. One electron, which has a negative charge, weighs only 1/1836 as much as the lightest of all nuclei, that of the hydrogen atom (proton). In addition, all the particles (pro ...
elements of chemistry unit
... Sometimes it is useful to assign oxidation numbers to elements found in polar covalent species. By creating Lewis Dot Structures (LDS) diagrams for each element, it is possible to determine their oxidation numbers. Next, combine the LDS diagrams for the elements and determine which electrons are sha ...
... Sometimes it is useful to assign oxidation numbers to elements found in polar covalent species. By creating Lewis Dot Structures (LDS) diagrams for each element, it is possible to determine their oxidation numbers. Next, combine the LDS diagrams for the elements and determine which electrons are sha ...
Balancing Reaction Equations Oxidation State Reduction
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
... Oxidation: Loss of electrons from an element. Oxidation number increases Reduction: Gain of electrons by an element. Oxidation number decreases ...
Campbell Biology, 10e (Reece) Chapter 2 The Chemical Context of
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
... B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element 6) In what way are elements in the same column of the periodic table the same? They have the same number of _____. A) protons B) electrons when neutral ...
Midterm Review Teacher Answer Key December 21, 2011 `see
... a. One point is awarded for drawing an appropriate scale on the y-axis. An appropriate scale is one that is large enough for a trend to be seen. [1 point] b. One point is awarded for plotting all four points correctly (within ±0.3 of a grid space). [1 point] c. Refer to the Periodic Table of the Ele ...
... a. One point is awarded for drawing an appropriate scale on the y-axis. An appropriate scale is one that is large enough for a trend to be seen. [1 point] b. One point is awarded for plotting all four points correctly (within ±0.3 of a grid space). [1 point] c. Refer to the Periodic Table of the Ele ...
The Periodic Table
... The atomic number (Z) is the number of protons in the nucleus. Atoms are neutral, so it’s also the number of electrons. Protons determine the identity of an element. For example, nitrogen’s atomic number is 7, so every nitrogen has 7 protons. The mass number (A) is the total number of protons and ...
... The atomic number (Z) is the number of protons in the nucleus. Atoms are neutral, so it’s also the number of electrons. Protons determine the identity of an element. For example, nitrogen’s atomic number is 7, so every nitrogen has 7 protons. The mass number (A) is the total number of protons and ...
Document
... • Believed you would never end up with an indivisible particle. • (no such thing as a smallest particle) ...
... • Believed you would never end up with an indivisible particle. • (no such thing as a smallest particle) ...
Atomic Theory - Alvinisd.net
... there is a tiny dense region (nucleus) in the center of an atom the nucleus contains most of the atom’s mass positive charges are found in the nucleus (Be sure to write these down. The results/analysis/conclusions are the most important parts of an experiment.) ...
... there is a tiny dense region (nucleus) in the center of an atom the nucleus contains most of the atom’s mass positive charges are found in the nucleus (Be sure to write these down. The results/analysis/conclusions are the most important parts of an experiment.) ...
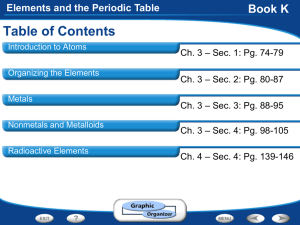
Elements and the Periodic Table
... • Group 15, the nitrogen family, contains two nonmetals: nitrogen and phosphorus. These non-metals usually gain or share three electrons when reacting with atoms of other elements. • Nitrogen is an example of an element that occurs in nature in the form of diatomic molecules, as N2. • A diatomic mol ...
... • Group 15, the nitrogen family, contains two nonmetals: nitrogen and phosphorus. These non-metals usually gain or share three electrons when reacting with atoms of other elements. • Nitrogen is an example of an element that occurs in nature in the form of diatomic molecules, as N2. • A diatomic mol ...
File
... 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; contains protons and neutrons 7) Formed the atomic theory model of the atom; English schoolteacher 8) Discovered the nucleus using his gold foil experiment 9) Current explanation of where electrons might be foun ...
... 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; contains protons and neutrons 7) Formed the atomic theory model of the atom; English schoolteacher 8) Discovered the nucleus using his gold foil experiment 9) Current explanation of where electrons might be foun ...
Name: Per: Date: Unit 1. Materials: Formulating Matter B. Periodic
... 38. Fill in the data table for each ionic compound described below. Number one is filled in as an example. Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO ...
... 38. Fill in the data table for each ionic compound described below. Number one is filled in as an example. Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO ...
Review # 3
... Schrodinger’s Quantum Mechanical Model: two principles (Pauli Exclusion and Aufbau) and a rule (Hund’s) Ground state electron configurations, based on which valence shell electron configuration, Lewis dot structure are established ...
... Schrodinger’s Quantum Mechanical Model: two principles (Pauli Exclusion and Aufbau) and a rule (Hund’s) Ground state electron configurations, based on which valence shell electron configuration, Lewis dot structure are established ...