Atoms - Issaquah Connect
... • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Atomic Structure - Miami East Schools
... 1. Matter is composed of tiny indivisible atoms 2. All atoms of a given element are exactly the same 3. Different elements are made of different atoms 4. Atoms of different elements can combine in simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or re ...
... 1. Matter is composed of tiny indivisible atoms 2. All atoms of a given element are exactly the same 3. Different elements are made of different atoms 4. Atoms of different elements can combine in simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or re ...
Chapter 4 and 25 Study Guide
... 16. What is the relative mass of protons, neutrons, and electrons? Protons 1; Neutrons 1; electrons about zero 17. Are atoms positive, negative, or neutral? How does the number of protons relate to the number of electrons? Atoms are neutral: number of protons and electrons in atoms are always equal ...
... 16. What is the relative mass of protons, neutrons, and electrons? Protons 1; Neutrons 1; electrons about zero 17. Are atoms positive, negative, or neutral? How does the number of protons relate to the number of electrons? Atoms are neutral: number of protons and electrons in atoms are always equal ...
Atomic Structure
... Isotopes of elements All atoms can be characterized by two numbers: the atomic number and the mass number. The atomic number (Z) is simply equal to the number of protons in the nucleus of the atom. As atoms are electrically neutral this will also be equal to the number of electrons in the atom. Ato ...
... Isotopes of elements All atoms can be characterized by two numbers: the atomic number and the mass number. The atomic number (Z) is simply equal to the number of protons in the nucleus of the atom. As atoms are electrically neutral this will also be equal to the number of electrons in the atom. Ato ...
PVS103 - unit 6 notes
... Periodic Properties of the Elements Groups 1a & 2a Metals and Non-metals Groups 3a to 8a; the Non-metals Groups 3b to 12b; the Transition Metals ...
... Periodic Properties of the Elements Groups 1a & 2a Metals and Non-metals Groups 3a to 8a; the Non-metals Groups 3b to 12b; the Transition Metals ...
Chemistry Study Guide
... Identify the choice that best completes the statement or answers the question. ____ 46. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 47. The study of chemicals that, in general, do not contain carbon is traditionally calle ...
... Identify the choice that best completes the statement or answers the question. ____ 46. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 47. The study of chemicals that, in general, do not contain carbon is traditionally calle ...
Chemistry Study Guide
... Identify the choice that best completes the statement or answers the question. ____ 46. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 47. The study of chemicals that, in general, do not contain carbon is traditionally calle ...
... Identify the choice that best completes the statement or answers the question. ____ 46. Which field of science studies the composition and structure of matter? a. physics c. chemistry b. biology d. geology ____ 47. The study of chemicals that, in general, do not contain carbon is traditionally calle ...
Topic exploration pack
... Because of the nature of the topic, many more advanced experiments are not particularly safe in a classroom environment, and half-lives and models of decay rates should already have been dealt with sufficiently in the main body of the course, so the activities provided are of a largely qualitative n ...
... Because of the nature of the topic, many more advanced experiments are not particularly safe in a classroom environment, and half-lives and models of decay rates should already have been dealt with sufficiently in the main body of the course, so the activities provided are of a largely qualitative n ...
Chapter #4 Section Assessment #1 - 33
... Is everything he said here still believed to be true? ii) Atoms of the same element are identical. The atoms of any one element are different from those of another element. Is everything he said here still believed to be true? iii) Atoms of different elements can physically mix together or can chemi ...
... Is everything he said here still believed to be true? ii) Atoms of the same element are identical. The atoms of any one element are different from those of another element. Is everything he said here still believed to be true? iii) Atoms of different elements can physically mix together or can chemi ...
Unit 03 Packet - Whitwell High School
... nucleus of an atom. The atom is __________ because this is also the number of __________ charged __________ in the atom. 2. The mass number tells the total number of________ and _________ in the nucleus of an atom. These particles collectively are called ___________ since both are located in the nuc ...
... nucleus of an atom. The atom is __________ because this is also the number of __________ charged __________ in the atom. 2. The mass number tells the total number of________ and _________ in the nucleus of an atom. These particles collectively are called ___________ since both are located in the nuc ...
Slide 1
... Heisenberg Uncertainty Principle • In 1927, Werner Heisenberg stated that it is impossible to simultaneously determine both the position and velocity of an electron or any other particle. This became known as the Heisenberg Uncertainty Principle. • Quantum theory was more widely accepted after this ...
... Heisenberg Uncertainty Principle • In 1927, Werner Heisenberg stated that it is impossible to simultaneously determine both the position and velocity of an electron or any other particle. This became known as the Heisenberg Uncertainty Principle. • Quantum theory was more widely accepted after this ...
Introductory Chemistry I
... 4. The maximum number of electrons that can occupy the 3d orbitals is a. 5 b. 6 c. 10 d. 14 e. 18 5. Let’s say that you are examining the outermost electrons in a ground-state germanium atom. Which of the following sets of values for the four quantum numbers (n, l, ml, and ms) could you use to descr ...
... 4. The maximum number of electrons that can occupy the 3d orbitals is a. 5 b. 6 c. 10 d. 14 e. 18 5. Let’s say that you are examining the outermost electrons in a ground-state germanium atom. Which of the following sets of values for the four quantum numbers (n, l, ml, and ms) could you use to descr ...
Ch 2 PowerPoint
... • All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements • Atoms of an element are not changed into atoms of a different by chemical rxns; atoms are neither created nor destroyed in chemi ...
... • All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements • Atoms of an element are not changed into atoms of a different by chemical rxns; atoms are neither created nor destroyed in chemi ...
File
... But where had these tiny particles come from? Since they were so small, Thomson suggested that they could only have come from inside atoms. So Dalton's idea of the indestructible atom had to be revised. Thomson proposed a different model for the atom. He said that the tiny negatively charged electro ...
... But where had these tiny particles come from? Since they were so small, Thomson suggested that they could only have come from inside atoms. So Dalton's idea of the indestructible atom had to be revised. Thomson proposed a different model for the atom. He said that the tiny negatively charged electro ...
Solutions 1a (suggested problems before Exam #1) Chem151 [Kua
... Molecular pictures must show the structures of individual particles (e.g., atoms, molecules, etc.) and the differences between phases. All these particles have monatomic units. A gas is mostly empty space, a liquid is tightly packed but not entirely regular, and a solid has a regular repeating struc ...
... Molecular pictures must show the structures of individual particles (e.g., atoms, molecules, etc.) and the differences between phases. All these particles have monatomic units. A gas is mostly empty space, a liquid is tightly packed but not entirely regular, and a solid has a regular repeating struc ...
Sorenson, Ch.1
... atom (electroncloud) is mostly empty space. 2. Terminology and Notation An atomic nucleus is characterized by the number of neutrons and protons it contains. The number of protons determines the atomic number of the atom, Z. As mentioned earlier, this determines also the number of orbital electrons ...
... atom (electroncloud) is mostly empty space. 2. Terminology and Notation An atomic nucleus is characterized by the number of neutrons and protons it contains. The number of protons determines the atomic number of the atom, Z. As mentioned earlier, this determines also the number of orbital electrons ...
Fall 2008 Blank Exam 1 - Department of Chemistry | Oregon State
... Fill in the front page of the Scantron answer sheet with your test form number (listed above), last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank. This exam consists of 25 multiple-choice questions. Each question ha ...
... Fill in the front page of the Scantron answer sheet with your test form number (listed above), last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank. This exam consists of 25 multiple-choice questions. Each question ha ...
Chemistry can be defined as the study of the composition, structure
... Since there no reserve store of sodium ions in the animal body, losses above the amount of intake come from the functional supply of cells and tissues. Salt (sodium chloride) is important in many ways. It is an essential part of the diet of both humans and animals and is a part of most animal fluids ...
... Since there no reserve store of sodium ions in the animal body, losses above the amount of intake come from the functional supply of cells and tissues. Salt (sodium chloride) is important in many ways. It is an essential part of the diet of both humans and animals and is a part of most animal fluids ...
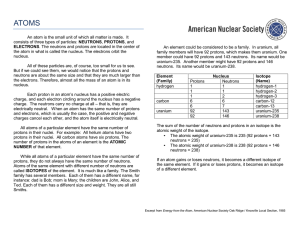
An atom is the small unit of which all matter is made. It consists of
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...