ChemistryReview
... 73. If an atom of tin has a mass number of 118 and an atomic number of 50, how many neutrons are in its nucleus? 74. In a periodic table that included electron dot diagrams, in which column would the diagrams contain more dots— Group 2A (the alkaline metals) or Group 6A (the oxygen family)? 75. In a ...
... 73. If an atom of tin has a mass number of 118 and an atomic number of 50, how many neutrons are in its nucleus? 74. In a periodic table that included electron dot diagrams, in which column would the diagrams contain more dots— Group 2A (the alkaline metals) or Group 6A (the oxygen family)? 75. In a ...
Chapter One
... order to cook food. But even the most liberal interpretation would not allow us to call this chemistry because of the absence of any evidence of control over these reactions or processes. The ability to control the transformation of one substance into another can be traced back to the origin of two ...
... order to cook food. But even the most liberal interpretation would not allow us to call this chemistry because of the absence of any evidence of control over these reactions or processes. The ability to control the transformation of one substance into another can be traced back to the origin of two ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
FREE Sample Here
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
Final Exam - KFUPM Faculty List
... In CO2 there are 2 CO σ-bonds, 2 CO π-bonds and 4 lone pairs, 2 on each oxygen. At each oxygen the σ-pair structure is formed by a triangle made up from the CO σ-bond and the 2 lone pairs. For these 3 electron pairs on each oxygen three hybrid orbitals are needed and thus an sp2 hybrid on each oxyge ...
... In CO2 there are 2 CO σ-bonds, 2 CO π-bonds and 4 lone pairs, 2 on each oxygen. At each oxygen the σ-pair structure is formed by a triangle made up from the CO σ-bond and the 2 lone pairs. For these 3 electron pairs on each oxygen three hybrid orbitals are needed and thus an sp2 hybrid on each oxyge ...
Chapter 2 13ed
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
TEKS Presentation Properties of Matter
... retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
... retaining heat is important to our climate. It means that our climate stays much more stable than it would if there were less water on Earth. TAKS Need to Know ...
Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
Chapter 2 - Woodhaven High School
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
... If two elements, A and B, form more than one compound, the masses of B that combine with a given mass of A are in the ratio of small whole numbers. Dalton predicted this law and observed it while developing his atomic theory. When two or more compounds exist from the same elements, they can not h ...
what`s ahead - Al Akhawayn University
... The Nuclear Model of the Atom With growing evidence that the atom is composed of smaller particles, attention was given to how the particles fit together. During the early 1900s, Thomson reasoned that because electrons contribute only a very small fraction of an atom’s mass they probably were respon ...
... The Nuclear Model of the Atom With growing evidence that the atom is composed of smaller particles, attention was given to how the particles fit together. During the early 1900s, Thomson reasoned that because electrons contribute only a very small fraction of an atom’s mass they probably were respon ...
Course Map_2011-2012 - Kenwood Academy High School
... elements are: 1. alkali metals, 2. alkaline Earth metals, 3. transition metals, 4. nonmetals, (5) metalloids, and (6) rare Earth elements. Know why hydrogen is not in any of these groups. 12.11.42 Know that there are two major different kinds of bonds (ionic and covalent). Know the distinction betwe ...
... elements are: 1. alkali metals, 2. alkaline Earth metals, 3. transition metals, 4. nonmetals, (5) metalloids, and (6) rare Earth elements. Know why hydrogen is not in any of these groups. 12.11.42 Know that there are two major different kinds of bonds (ionic and covalent). Know the distinction betwe ...
WRL0001.tmp - Ethiopian Teachers Association
... lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory experiments, use of structural models and technology-based methods. Chemistry is one of the most important ...
... lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory experiments, use of structural models and technology-based methods. Chemistry is one of the most important ...
Chapter 2: Atoms, Ions, and the Periodic Table
... C) nonmetals Ans: B 58. Which of the following statements does not apply to metalloids? A) The physical properties of metalloids resemble those of a metal. B) All metalloids are electrical insulators. C) Metalloids lie along the stair-step line beginning at boron. D) The chemical properties of metal ...
... C) nonmetals Ans: B 58. Which of the following statements does not apply to metalloids? A) The physical properties of metalloids resemble those of a metal. B) All metalloids are electrical insulators. C) Metalloids lie along the stair-step line beginning at boron. D) The chemical properties of metal ...
Chapter 2: Atoms, Ions, and the Periodic Table
... C) nonmetals Ans: B 58. Which of the following statements does not apply to metalloids? A) The physical properties of metalloids resemble those of a metal. B) All metalloids are electrical insulators. C) Metalloids lie along the stair-step line beginning at boron. D) The chemical properties of metal ...
... C) nonmetals Ans: B 58. Which of the following statements does not apply to metalloids? A) The physical properties of metalloids resemble those of a metal. B) All metalloids are electrical insulators. C) Metalloids lie along the stair-step line beginning at boron. D) The chemical properties of metal ...
Chapter 3 - SaddleSpace/Haiku
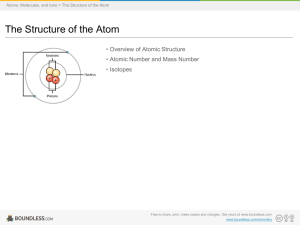
... V. Properties of Atoms and Ions atomic number (Z): number of protons in an atom mass number: number of protons + neutrons in an atom (number of nucleons— particles in the nucleus) isotopes (nuclides): atoms of the same ...
... V. Properties of Atoms and Ions atomic number (Z): number of protons in an atom mass number: number of protons + neutrons in an atom (number of nucleons— particles in the nucleus) isotopes (nuclides): atoms of the same ...
Chapter 2 - San Joaquin Memorial High School
... Lavoisier’s quantitative experiments showed that combustion involved oxygen (which Lavoisier named), not phlogiston. He also discovered that life was supported by a process that also involved oxygen and was similar in many ways to combustion. In 1789 Lavoisier published the first modern chemistry te ...
... Lavoisier’s quantitative experiments showed that combustion involved oxygen (which Lavoisier named), not phlogiston. He also discovered that life was supported by a process that also involved oxygen and was similar in many ways to combustion. In 1789 Lavoisier published the first modern chemistry te ...
Boundless Study Slides
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
... • Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties. • Some isotopes are unstable and will undergo radioactive decay to become other elements. • The predictable half-life of different decaying isotopes allows scientists to date material ...
Chapter 2: Matter Is Made up of Atoms
... experiments. For example, Dalton’s atomic theory was based on observations of matter performed again and again by many scientists. As scientists gather more information, a theory may have to be revised or replaced with another theory. In nonscientific speech and writing, the word theory is often use ...
... experiments. For example, Dalton’s atomic theory was based on observations of matter performed again and again by many scientists. As scientists gather more information, a theory may have to be revised or replaced with another theory. In nonscientific speech and writing, the word theory is often use ...
Atomic Structure Practice Test
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
... ____ 10. The smallest unit of an element that can exist either alone or in combination with other such particles of the same or different elements is the a. electron. b. proton. c. neutron. d. atom. ____ 11. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus. c. ...
Ch.1-Matter and Change
... Elements Types of Elements Metals A metal is an element that is good electrical conductor and a good heat conductor. Properties of Metals most are solid at room temperature malleable - they can be hammered or rolled into thin sheets ductile - they can be drawn into a thin wire conduct electricity a ...
... Elements Types of Elements Metals A metal is an element that is good electrical conductor and a good heat conductor. Properties of Metals most are solid at room temperature malleable - they can be hammered or rolled into thin sheets ductile - they can be drawn into a thin wire conduct electricity a ...
1 Atomic Orbital Theory
... model of the atom postulated by Niels Bohr (1885–1962), electrons surrounding the nucleus are placed in circular orbits. The electrons move in these orbits much as planets orbit the sun. In rationalizing atomic emission spectra of the hydrogen atom, Bohr assumed that the energy of the electron in di ...
... model of the atom postulated by Niels Bohr (1885–1962), electrons surrounding the nucleus are placed in circular orbits. The electrons move in these orbits much as planets orbit the sun. In rationalizing atomic emission spectra of the hydrogen atom, Bohr assumed that the energy of the electron in di ...