- TestbankU
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
Document
... that the atom had pieces called electrons • Thomson found that electrons are much smaller than atoms and carry a negative charge the mass of the electron is 1/1836th the mass of a hydrogen atom the charge on the electron is the fundamental unit of charge which we will call –1 charge units Tro's In ...
... that the atom had pieces called electrons • Thomson found that electrons are much smaller than atoms and carry a negative charge the mass of the electron is 1/1836th the mass of a hydrogen atom the charge on the electron is the fundamental unit of charge which we will call –1 charge units Tro's In ...
Chapter 3 - WordPress.com
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
Beginning Chemistry
... Compounds are substances consisting of two or more elements combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydroge ...
... Compounds are substances consisting of two or more elements combined in definite proportions by mass to give a material having a definite set of properties different from that of any of its constituent elements. For example, the compound water consists of 88.8 percent oxygen and 11.2 percent hydroge ...
Hewitt/Lyons/Suchocki/Yeh, Conceptual Integrated Science
... • to date, 115 are known – 90 occur in nature – others produced in laboratory are unstable Words atom and element can be used interchangeably Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley ...
... • to date, 115 are known – 90 occur in nature – others produced in laboratory are unstable Words atom and element can be used interchangeably Copyright © 2008 Pearson Education, Inc., publishing as Pearson Addison-Wesley ...
No Slide Title
... magazines? 2. Once you arrange the magazines into groups, could you sort the material further to make it even more organized? ...
... magazines? 2. Once you arrange the magazines into groups, could you sort the material further to make it even more organized? ...
Chapter 3
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
mc_ch03
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
... • When two protons are extremely close to each other, there is a strong attraction between them. • A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. • The short-range proton-neutron, proton-proton, and neutron-neutron forces ...
Chapter 2 ATOMS AND ELEMENTS
... Instructor: This PowerLecture contains two PowerPoint presentations: one with lecture content and art from the text, the other with videos and animations. For animations and videos to run properly, we recommend that you run this PowerPoint presentation from the PowerLecture disc inserted in your com ...
... Instructor: This PowerLecture contains two PowerPoint presentations: one with lecture content and art from the text, the other with videos and animations. For animations and videos to run properly, we recommend that you run this PowerPoint presentation from the PowerLecture disc inserted in your com ...
Chapter 20
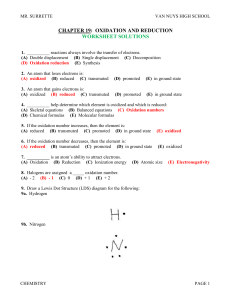
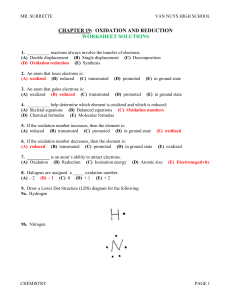
... Charcoal oxidizes when it burn forming CO2 Bleaching stains in fabric is still oxidation even though it does not burn. ...
... Charcoal oxidizes when it burn forming CO2 Bleaching stains in fabric is still oxidation even though it does not burn. ...
Chapter 22 - 2012 Book Archive
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
... Group 13 is the first group to span the dividing line between metals and nonmetals, so its chemistry is more diverse than that of groups 1 and 2, which include only metallic elements. Except for the lightest element (boron), the group 13 elements are all relatively electropositive; that is, they ten ...
FREE Sample Here
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
Lecture 2
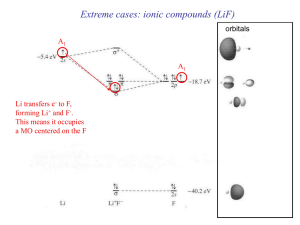
... SALC can now be treated similarly to the atomic orbitals and combined with appropriate AO’s from H 1s(H) is Ag so it matches two SALC. The interaction can be bonding or antibonding. ...
... SALC can now be treated similarly to the atomic orbitals and combined with appropriate AO’s from H 1s(H) is Ag so it matches two SALC. The interaction can be bonding or antibonding. ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Chemistry - Textbooks Online
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
... Chemistry, a branch of science concerned with the properties, structures and composition of substances and their reactions with one another. Inorganic Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry s ...
Atomic Theory and Periodic Table Review Multiple Choice Identify
... a. Protons, neutrons, and electrons all have about the same mass. b. Unlike protons or neutrons, electrons have no mass. c. Neutrons have no charge and no mass. d. An electron has far less mass than either a proton or neutron. 10. Which of the following is unique for any given element? a. the number ...
... a. Protons, neutrons, and electrons all have about the same mass. b. Unlike protons or neutrons, electrons have no mass. c. Neutrons have no charge and no mass. d. An electron has far less mass than either a proton or neutron. 10. Which of the following is unique for any given element? a. the number ...
FREE Sample Here
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...
... A) the number of electrons in the element B) the number of protons in the element C) the number of protons plus neutrons in the element D) the number of protons plus electrons in the element Answer: C Bloom's Taxonomy: Application/Analysis Section: 2.2 6) In what way are elements in the same column ...