Lecture 12 Quantum Mechanics and Atomic Orbitals Bohr and
... The 1s orbital has the electron closest to the nucleus, so it has the lowest energy.The 2s and 2p orbitals have the same energy for hydrogen. They are said to be degenerate energy levels, all the same. The n = 3 orbitals are the next highest in energy, followed by the degeneraten = 4 orbitals. When ...
... The 1s orbital has the electron closest to the nucleus, so it has the lowest energy.The 2s and 2p orbitals have the same energy for hydrogen. They are said to be degenerate energy levels, all the same. The n = 3 orbitals are the next highest in energy, followed by the degeneraten = 4 orbitals. When ...
2nd Semester Review
... 6. What causes a substance to change from one state to another?_______________________________ 7. Write physical or chemical change for each of the following: Water evaporating burning toast Fireworks exploding ice melting ...
... 6. What causes a substance to change from one state to another?_______________________________ 7. Write physical or chemical change for each of the following: Water evaporating burning toast Fireworks exploding ice melting ...
Regents Review Packet B2 Answer Key
... The equation below represents an equilibrium system of reaction can be catalyzed by vanadium or platinum. ...
... The equation below represents an equilibrium system of reaction can be catalyzed by vanadium or platinum. ...
Chapter 3: The Structure of Matter
... energy level •Electron dot diagrams (EDD) just the valence electrons ...
... energy level •Electron dot diagrams (EDD) just the valence electrons ...
File - Mr. Gittermann
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
... with no charge and is located in the nucleus of the atom • Electrons: Subatomic particle with a negative charge found in a certain region of space around the nucleus called the electron cloud; kept close to the atom due to the attraction between the opposite charges of the electron and proton ...
Study Guide Matter: Building Blocks of the Universe
... * Know the atomic particles: electron, neutron, and proton. where are they in the atom? What is their charge? What is their mass? How are electrons arranged in the electron cloud? * Know the four forces in the atom: strong, electromagnetic, weak, & gravity What are each of the forces responsible for ...
... * Know the atomic particles: electron, neutron, and proton. where are they in the atom? What is their charge? What is their mass? How are electrons arranged in the electron cloud? * Know the four forces in the atom: strong, electromagnetic, weak, & gravity What are each of the forces responsible for ...
Review 3rd Qtr KEY
... 0.0010 has __2__ significant figures & rounded to 3 significant figures is 0.00100 or 1.00 x 10-3 3.5 x 1023 has __2___ significant figures & rounded to 4 significant figures is 3.500 x 1023 ...
... 0.0010 has __2__ significant figures & rounded to 3 significant figures is 0.00100 or 1.00 x 10-3 3.5 x 1023 has __2___ significant figures & rounded to 4 significant figures is 3.500 x 1023 ...
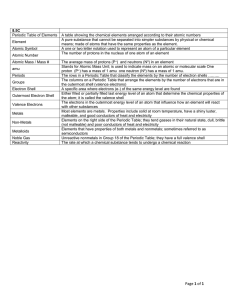
8.5C Vocabulary
... The columns on a Periodic Table that arrange the elements by the number of electrons that are in the outermost shell (valence electrons) A specific area where electrons (e-) of the same energy level are found Either filled or partially filled last energy level of an atom that determine the chemical ...
... The columns on a Periodic Table that arrange the elements by the number of electrons that are in the outermost shell (valence electrons) A specific area where electrons (e-) of the same energy level are found Either filled or partially filled last energy level of an atom that determine the chemical ...
1 - M*W
... d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They want to lose an electron to complete their outer energy level c) They want to gain a proton in their nucleus d) They want to lose a proton from their nucleus ...
... d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They want to lose an electron to complete their outer energy level c) They want to gain a proton in their nucleus d) They want to lose a proton from their nucleus ...
Problem
... • The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers n, l, ml and ms. – For a given orbital the values of n, l, and ml ...
... • The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers n, l, ml and ms. – For a given orbital the values of n, l, and ml ...
3UE-Exam Review-June2010 - Savita Pall and Chemistry
... 19. When is ionic bonding likely to occur between two atoms? a) when both atoms have low ionization energy and low electron affinity b) when both atoms have high ionization energy and low electron affinity c) when both atoms have high ionization energy and high electron affinity d) when one atom has ...
... 19. When is ionic bonding likely to occur between two atoms? a) when both atoms have low ionization energy and low electron affinity b) when both atoms have high ionization energy and low electron affinity c) when both atoms have high ionization energy and high electron affinity d) when one atom has ...
Note 1.1 Chemistry of Life
... be an unequal sharing between these atoms. The greater the electronegativity that atom has, the greater the attraction to an electron from another atom. The unequal sharing of electrons between two atoms, with two different electronegativities, will result in a polar covalent bond. An atom which att ...
... be an unequal sharing between these atoms. The greater the electronegativity that atom has, the greater the attraction to an electron from another atom. The unequal sharing of electrons between two atoms, with two different electronegativities, will result in a polar covalent bond. An atom which att ...
Notes 7
... OVERALL IDEA of Hartree technique is that any one electron moves in a potential which is a spherical average of the potential due to all the other electrons and the nucleus, wand this is expressed as a single charge centered on the nucleus. (This is the central field approximation; but it is not ass ...
... OVERALL IDEA of Hartree technique is that any one electron moves in a potential which is a spherical average of the potential due to all the other electrons and the nucleus, wand this is expressed as a single charge centered on the nucleus. (This is the central field approximation; but it is not ass ...
Chapter 9. Molecular Geometry and Bonding Theories
... • Lewis structures and VSEPR theory give us the shape and location of electrons in a molecule. • They do not explain why a chemical bond forms. • How can quantum mechanics be used to account for molecular shape? What are the orbitals that are involved in bonding? • We use valence-bond theory. • A co ...
... • Lewis structures and VSEPR theory give us the shape and location of electrons in a molecule. • They do not explain why a chemical bond forms. • How can quantum mechanics be used to account for molecular shape? What are the orbitals that are involved in bonding? • We use valence-bond theory. • A co ...
Exam 3 Review
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
Quantum Numbers
... • The p sublevel has three orbitals, each of which can hold 2 electrons. That is why there are six columns in the “p” block. • The d sublevel has five orbitals, each of which can hold 2 electrons. That is why there are ten columns in the ...
... • The p sublevel has three orbitals, each of which can hold 2 electrons. That is why there are six columns in the “p” block. • The d sublevel has five orbitals, each of which can hold 2 electrons. That is why there are ten columns in the ...
First Semester Final - Review Questions
... 37. Describe the different amounts and kinds of damage in matter produced by the different penetrations of each type of radioactive decay. 38. How does the energy release in a nuclear reaction compare to the energy release in a chemical reaction. Investigation and Experimentation 39. What is the pur ...
... 37. Describe the different amounts and kinds of damage in matter produced by the different penetrations of each type of radioactive decay. 38. How does the energy release in a nuclear reaction compare to the energy release in a chemical reaction. Investigation and Experimentation 39. What is the pur ...
Basic Chemistry notes
... ______________________—two or more like atoms combined chemically ______________________—two or more different atoms combined chemically ...
... ______________________—two or more like atoms combined chemically ______________________—two or more different atoms combined chemically ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.