Atoms
... metals, alkaline earth metals, halogens, noble gases, lanthanide series, actinide series, transition elements, inner-transition elements, and trans-uranic elements. 6. Identify the phase (solid, liquid, gas) of each element on the Periodic Table. 7. Explain the basic periodic trends of radius and io ...
... metals, alkaline earth metals, halogens, noble gases, lanthanide series, actinide series, transition elements, inner-transition elements, and trans-uranic elements. 6. Identify the phase (solid, liquid, gas) of each element on the Periodic Table. 7. Explain the basic periodic trends of radius and io ...
Energy Level Models - Middle School Chemistry
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
... As the note on page 292 points out, there are other ways to model the electron energy levels of atoms. Some middle school texts show the electrons in pairs on an energy level. This pairing of electrons is intended to suggest information about the substructure within energy levels. This substructure ...
An element`s properties depend on the structure of its atoms
... • In the 1930’s Linus Pauling introduced the concept of hybridization to explain chemical bond formation. Hybridization is the mixing of atomic orbitals in an atom to generate a set of new atomic orbitals called hybrid orbitals. • Mixing an s orbital with one of the p orbitals generates two equivale ...
... • In the 1930’s Linus Pauling introduced the concept of hybridization to explain chemical bond formation. Hybridization is the mixing of atomic orbitals in an atom to generate a set of new atomic orbitals called hybrid orbitals. • Mixing an s orbital with one of the p orbitals generates two equivale ...
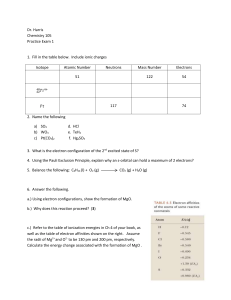
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 12. A certain element, X, has 3 isotopes (49X, 50X, and 51X). The isotope masses are 49.122 amu, 50.456 amu, and 51.246 amu. Analysis confirms that 3.00% of all X is 51X. Given that the average mass of X is 50.000, determine the percent abundances of the other isotopes. ...
... 12. A certain element, X, has 3 isotopes (49X, 50X, and 51X). The isotope masses are 49.122 amu, 50.456 amu, and 51.246 amu. Analysis confirms that 3.00% of all X is 51X. Given that the average mass of X is 50.000, determine the percent abundances of the other isotopes. ...
Review-Semester Final (Part I)
... 41. Put Sr, Cr, Fr, Al, F, Cs and P in order of: a. Largest to smallest atomic radius:____________________________________________ b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. ...
... 41. Put Sr, Cr, Fr, Al, F, Cs and P in order of: a. Largest to smallest atomic radius:____________________________________________ b. Largest to smallest electronegativity:________________________________________ c. Largest to smallest ionization energy:_________________________________________ 42. ...
Atoms and Materials for Engineering
... entry. We only know that they are second-level p orbitals (2p). The number 4 after the p indicates that there are 4 electrons in 2p orbitals. It is expected that we already know there are a maximum of three p orbitals at level 2, two contain Figure 5 oxygen one electron each and the other has 2 elec ...
... entry. We only know that they are second-level p orbitals (2p). The number 4 after the p indicates that there are 4 electrons in 2p orbitals. It is expected that we already know there are a maximum of three p orbitals at level 2, two contain Figure 5 oxygen one electron each and the other has 2 elec ...
Honors Chemistry Semester 1 Exam Review
... 9. Which diagram correctly depicts the trend in electronegativity? ________ 10. Which diagram correctly depicts the trend in atomic radius? ________ 11. Elements in the same group have similar ______________. They behave similarly because they have the same number of ________________. 12. Metals are ...
... 9. Which diagram correctly depicts the trend in electronegativity? ________ 10. Which diagram correctly depicts the trend in atomic radius? ________ 11. Elements in the same group have similar ______________. They behave similarly because they have the same number of ________________. 12. Metals are ...
The Periodic table
... A region of space within an electron subshell where an electron with a specific energy is most likely to be found. S subshell=1 orbital, p subshell=3 orbitals, d subshell=5 orbitals, f subshell=7 orbitals. Maximum number of electrons in a subshell is always 2. S orbital=spherical, p orbital ...
... A region of space within an electron subshell where an electron with a specific energy is most likely to be found. S subshell=1 orbital, p subshell=3 orbitals, d subshell=5 orbitals, f subshell=7 orbitals. Maximum number of electrons in a subshell is always 2. S orbital=spherical, p orbital ...
Test #5 Review
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
Name: Date: Chemistry 1 – Midterm Review Sheet Unit 1 – Scientific
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
... 3. The energy levels of the hydrogen atom (and all atoms) are ______________, meaning that only certain discrete energy levels are allowed. a. varied b. quantized c. ramp-like d. continuous e. two of these 4. The form of EMR that has less energy than microwaves is a. microwaves b. radio waves c. ga ...
The Chemical Basis of Life
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
... Isotopes of an element – Different forms of an element with the same atomic number but with different mass numbers – The atoms of some isotopes are stable – Other isotopes are radioactive, having unstable atoms that spontaneously break apart (decay) to form other atoms – When radioactive atoms decay ...
chemia simr01 en - Leszek Niedzicki
... obtaining fully occupied outermost electron subshell. Depending on the starting point - in which direction the target is closer - they can ‘accept’ (acceptor) electrons from other atoms or ‘donate’ (donor) electrons to the bond (share them). • Additionally, bonding is also beneficial energetically – ...
... obtaining fully occupied outermost electron subshell. Depending on the starting point - in which direction the target is closer - they can ‘accept’ (acceptor) electrons from other atoms or ‘donate’ (donor) electrons to the bond (share them). • Additionally, bonding is also beneficial energetically – ...
3rd Quarter Test
... a) a polar covalent bond with an electronegativity difference of zero b) a polar covalent bond with an electronegativity difference between zero and 1.7 c) a non-polar covalent bond with an electronegativity difference of zero d) a non-polar covalent bond with an electronegativity difference between ...
... a) a polar covalent bond with an electronegativity difference of zero b) a polar covalent bond with an electronegativity difference between zero and 1.7 c) a non-polar covalent bond with an electronegativity difference of zero d) a non-polar covalent bond with an electronegativity difference between ...
Odd Number of Electrons
... 2. Usually expressed as the energy needed to break one mole of bonds. 3. A large bond dissociation energy corresponds to a strong covalent bond. 4. High dissociation energies tend to create very stable compounds that tend to be chemically unreactive. 5. Units are measured in kJ/mo1 6. A mol is a che ...
... 2. Usually expressed as the energy needed to break one mole of bonds. 3. A large bond dissociation energy corresponds to a strong covalent bond. 4. High dissociation energies tend to create very stable compounds that tend to be chemically unreactive. 5. Units are measured in kJ/mo1 6. A mol is a che ...
Experiment S4
... NOTE: Much of this discussion would have been simpler if, in changing to SI units, GNS had written B for µoH, Additional Material for FIG.1 GNS (p.365) Co(III) has six 3d electrons. If the energy correlating each of these electrons with the others is neglected, the energy of each electron (called an ...
... NOTE: Much of this discussion would have been simpler if, in changing to SI units, GNS had written B for µoH, Additional Material for FIG.1 GNS (p.365) Co(III) has six 3d electrons. If the energy correlating each of these electrons with the others is neglected, the energy of each electron (called an ...
Activity 17 Follow-up
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
... very reactive. When the sodium reacts with the water it takes the place of one of the hydrogen atoms. This happens because sodium is more reactive than the hydrogen it is replacing. Reactivity is largely due to the atomic radius of an element and the valence. Larger metals lose their outer electrons ...
Table showing examples of Complex ions with their bond
... Coloured ions All except Sc group and Zn group, d-block elements have coloured ions. This is because each ion absorbs light of certain wavelengths only in the visible past of the spectrum, thus changing incident white light into light whose hue () is composed of the colours complementary those whic ...
... Coloured ions All except Sc group and Zn group, d-block elements have coloured ions. This is because each ion absorbs light of certain wavelengths only in the visible past of the spectrum, thus changing incident white light into light whose hue () is composed of the colours complementary those whic ...
Test Review # 2 - Evan`s Chemistry Corner
... of atoms with more electrons. The wave mechanical model solved the problem. Thinking of the electron as a standing wave also helps to explain why the electron’s energy is quantized. The wave mechanical model describes the location of electrons a their most probable location rather than as orbits wit ...
... of atoms with more electrons. The wave mechanical model solved the problem. Thinking of the electron as a standing wave also helps to explain why the electron’s energy is quantized. The wave mechanical model describes the location of electrons a their most probable location rather than as orbits wit ...
Molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular orbitals, although the electrons involved may be redistributed among the orbitals. This tool is very well suited for simple diatomic molecules such as dihydrogen, dioxygen, and carbon monoxide but becomes more complex when discussing even comparatively simple polyatomic molecules, such as methane. MO diagrams can explain why some molecules exist and others do not. They can also predict bond strength, as well as the electronic transitions that can take place.