THE STUDY OF INTERMEDIARY METABOLISM OF
... atoms. Many compounds when treated with hot concentrated DzSO4 exchange otherwise stable hydrogen atoms (59). A number of deuterium-containing fatty acids and amino acids have thus been prepared by this procedure (60, 61). The method introduces deuterium into fatty acids only at the a-carbon atom. A ...
... atoms. Many compounds when treated with hot concentrated DzSO4 exchange otherwise stable hydrogen atoms (59). A number of deuterium-containing fatty acids and amino acids have thus been prepared by this procedure (60, 61). The method introduces deuterium into fatty acids only at the a-carbon atom. A ...
Groups 2 and 7
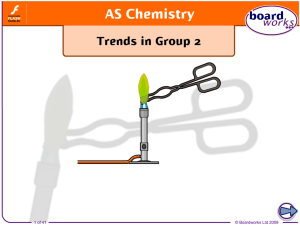
... When heated, the group 2 metal carbonates decompose to form the metal oxide and carbon dioxide gas. Splitting compounds using heat is called thermal decomposition. MCO3(s) MO(s) + CO2(g) ...
... When heated, the group 2 metal carbonates decompose to form the metal oxide and carbon dioxide gas. Splitting compounds using heat is called thermal decomposition. MCO3(s) MO(s) + CO2(g) ...
couverture these PRES Toulouse M ESCARCEGA 2011
... catalysts as “homogeneous”, relating it to its possessing only a single type of active site and if having many active sites as “heterogeneous” catalysts.10 In particular, homogeneous asymmetric catalysis provides a powerful tool for the synthesis of optically active molecules such as fine chemicals ...
... catalysts as “homogeneous”, relating it to its possessing only a single type of active site and if having many active sites as “heterogeneous” catalysts.10 In particular, homogeneous asymmetric catalysis provides a powerful tool for the synthesis of optically active molecules such as fine chemicals ...
Reactants Products
... mole of I2 will also be used and 2 moles of HI made. – Therefore, the rate of change will be different. ...
... mole of I2 will also be used and 2 moles of HI made. – Therefore, the rate of change will be different. ...
AP CHEMISTRY 2005/2006
... The Lake Norman High School AP Chemistry course is designed to meet the requirements and curriculum of a year-long, two semester general chemistry course usually taken during the freshman year of college. The course gives the college freshmen second-year work in chemistry sequence at their instituti ...
... The Lake Norman High School AP Chemistry course is designed to meet the requirements and curriculum of a year-long, two semester general chemistry course usually taken during the freshman year of college. The course gives the college freshmen second-year work in chemistry sequence at their instituti ...
UILChemistryProblemsPart2
... H2 (g) + CO (g) has the numerical value 0.50 at 600C. A mixture of HCHO, H2, and CO is introduced into a flask at 600C. After a short time, analysis of a small sample of the reaction mixture shows the concentration to be [HCHO] = 1.5, [H2] = 0.5 and [CO] = 1.0 moles/liter. Which of the following ...
... H2 (g) + CO (g) has the numerical value 0.50 at 600C. A mixture of HCHO, H2, and CO is introduced into a flask at 600C. After a short time, analysis of a small sample of the reaction mixture shows the concentration to be [HCHO] = 1.5, [H2] = 0.5 and [CO] = 1.0 moles/liter. Which of the following ...
Kinetic modelling of the Maillard reaction between proteins and sugars
... Another type of non-enzymatic browning occurring during heating is caramelisation, a complex process in which sugar reaction products condense and form brown coloured macromolecules. Although the Maillard reaction as a sugar-amine reaction should be distinguished from the caramelisation reaction occ ...
... Another type of non-enzymatic browning occurring during heating is caramelisation, a complex process in which sugar reaction products condense and form brown coloured macromolecules. Although the Maillard reaction as a sugar-amine reaction should be distinguished from the caramelisation reaction occ ...
EXAM IIR - Academics
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
Gas Laws
... 15. Write a balance equation and label the states of the reactants and products for each the reactions. After writing the balanced equation, write the net ionic equations below the balanced equation. If there is no driving force for a reaction, write no reaction in place of the net ionic equation. ...
... 15. Write a balance equation and label the states of the reactants and products for each the reactions. After writing the balanced equation, write the net ionic equations below the balanced equation. If there is no driving force for a reaction, write no reaction in place of the net ionic equation. ...
Problem 1: “A brief history” of life in the universe
... Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different spectroscopic methods. Absorption spectra of inters ...
... Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different spectroscopic methods. Absorption spectra of inters ...
chemical kinetics type 1.mdi
... Photochemical reactions. Those reactions which take place in the presence of light are called photochemical reactions. Photosynthesis is an example of photochemical reaction. Photosensitization. The process in which a molecule that absorbs light transfers its extra energy to another molecule which m ...
... Photochemical reactions. Those reactions which take place in the presence of light are called photochemical reactions. Photosynthesis is an example of photochemical reaction. Photosensitization. The process in which a molecule that absorbs light transfers its extra energy to another molecule which m ...
2015 Dr. Jay L. Wile, All rights reserved.
... Chapter 2 Comprehension Check Questions 1. Classify the following as a mixture or a pure substance: a. A bowl of cereal and milk b. An ice cube c. A drink made by dissolving a powder in water d. A sample of oxygen 2. Classify the following mixtures as heterogeneous or homogeneous: a. A bowl of cerea ...
... Chapter 2 Comprehension Check Questions 1. Classify the following as a mixture or a pure substance: a. A bowl of cereal and milk b. An ice cube c. A drink made by dissolving a powder in water d. A sample of oxygen 2. Classify the following mixtures as heterogeneous or homogeneous: a. A bowl of cerea ...
Problem 1: A brief history of life in the universe
... Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different spectroscopic methods. Absorption spectra of inters ...
... Atoms in interstellar space seldom meet. When they do (most likely on ice surfaces), they produce radicals and molecules. These species, some of which presumably played a role in the origin of life, have been identified through the use of different spectroscopic methods. Absorption spectra of inters ...
The Chemistry of Aqueous Systems
... 54) Be able to explain, describe and illustrate the various phases in a chromatography column; 55) Be able to explain the difference between a solution and a colloid; 56) Be able to explain what a solvent and a dispersing medium are and the differences between them; 57) Be able to explain what a sol ...
... 54) Be able to explain, describe and illustrate the various phases in a chromatography column; 55) Be able to explain the difference between a solution and a colloid; 56) Be able to explain what a solvent and a dispersing medium are and the differences between them; 57) Be able to explain what a sol ...
File
... Sustainable chemistry requires chemists to design processes with high atom economy that minimise production of waste products. ...
... Sustainable chemistry requires chemists to design processes with high atom economy that minimise production of waste products. ...
Unit-2-Hydrocarbons
... Organic chemistry is the chemistry of carbon. The name “organic” reflect the fact that organic molecules are derived from living organisms. In this unit will start by looking at four families of organic molecules that are grouped together as the hydrocarbons. We will also look at some functional gro ...
... Organic chemistry is the chemistry of carbon. The name “organic” reflect the fact that organic molecules are derived from living organisms. In this unit will start by looking at four families of organic molecules that are grouped together as the hydrocarbons. We will also look at some functional gro ...
CHAPTER 16
... If a large amount of energy as heat is released when a compound is formed, the compound has a large negative enthalpy of formation. Such compounds are very stable. Elements in their standard states are defined as having ∆H f0 = 0. The ∆H f0 of carbon dioxide is -393.5 kJ/mol of gas produced. Therefo ...
... If a large amount of energy as heat is released when a compound is formed, the compound has a large negative enthalpy of formation. Such compounds are very stable. Elements in their standard states are defined as having ∆H f0 = 0. The ∆H f0 of carbon dioxide is -393.5 kJ/mol of gas produced. Therefo ...
ΔG - Lemon Bay High School
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
Slide 1
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
Abdullah F. Eid
... polar solvents and fairly high thermal stability in the solid state. These properties render HPAs potentially promising acid, redox, and bifunctional catalysts in homogeneous as well as in heterogeneous systems. HPAs are widely used as model systems for fundamental research, providing unique opportu ...
... polar solvents and fairly high thermal stability in the solid state. These properties render HPAs potentially promising acid, redox, and bifunctional catalysts in homogeneous as well as in heterogeneous systems. HPAs are widely used as model systems for fundamental research, providing unique opportu ...
CHAPTER 15 ACIDS AND BASES
... The positive root of the equation is x = 8.6 × 10 M. (Is this less than 5% of the original concentration, 0.00020 M? That is, is the acid more than 5% ionized?) The percent ionization is then: ...
... The positive root of the equation is x = 8.6 × 10 M. (Is this less than 5% of the original concentration, 0.00020 M? That is, is the acid more than 5% ionized?) The percent ionization is then: ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.