Chapter 2 Notes - Duplin County Schools
... Mixture: a combination of substances in which the individual components retain their own properties Solution: a mixture in which one or more substances are distributed evenly in another substance ...
... Mixture: a combination of substances in which the individual components retain their own properties Solution: a mixture in which one or more substances are distributed evenly in another substance ...
Chapter 11 Chemical Reactions
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. How? We recognize them by their reactants ...
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. How? We recognize them by their reactants ...
Document
... 36. Peroxodisulfate ion can oxidize iodide ions to iodine according to the balanced equation S2O82- + 2I- ––> 2SO42- + I2. The reaction is catalyzed by certain chemical species. Identify the catalyst in the following mechanism: step 1: Fe3 + + 2I - ––> Fe2 + + I2 step 2: S2O82- + Fe2 + ––> 2SO42- + ...
... 36. Peroxodisulfate ion can oxidize iodide ions to iodine according to the balanced equation S2O82- + 2I- ––> 2SO42- + I2. The reaction is catalyzed by certain chemical species. Identify the catalyst in the following mechanism: step 1: Fe3 + + 2I - ––> Fe2 + + I2 step 2: S2O82- + Fe2 + ––> 2SO42- + ...
AP Chemistry Review Packet 1 CO2(g) + H2(g) « H2O(g) + CO(g
... (a) What equipment would be needed? (b) What measurements should be taken? (c) Without performing calculations, describe how the resulting data should be used to obtain the standard molar enthalpy of neutralization. (d) When a class of students performed this experiment, the average of the results w ...
... (a) What equipment would be needed? (b) What measurements should be taken? (c) Without performing calculations, describe how the resulting data should be used to obtain the standard molar enthalpy of neutralization. (d) When a class of students performed this experiment, the average of the results w ...
Chapter 11 Chemical Reactions
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
國立嘉義大學九十二學年度
... (A) The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature. (B) The molecules of an ideal gas are relatively far apart. (C) All molecules of an ideal gas have the same kinetic energy at constant temperature. (D) Molecules of a gas underg ...
... (A) The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature. (B) The molecules of an ideal gas are relatively far apart. (C) All molecules of an ideal gas have the same kinetic energy at constant temperature. (D) Molecules of a gas underg ...
An Overview of Organic Reactions
... by π electrons of ethylene (nucleophile) to form a carbocation intermediate and bromide ion Bromide adds to the positive center of the carbocation, which is an electrophile, forming a C-Br σ bond The result is that ethylene and HBr combine to form bromoethane All polar reactions occur by combination ...
... by π electrons of ethylene (nucleophile) to form a carbocation intermediate and bromide ion Bromide adds to the positive center of the carbocation, which is an electrophile, forming a C-Br σ bond The result is that ethylene and HBr combine to form bromoethane All polar reactions occur by combination ...
Chapter 11 Chemical Reactions
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
Chapter 11 Chemical Reactions
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
... We will learn: a) the 5 major types. We will be able to: b) predict the products. For some, we will be able to: c) predict whether or not they will happen at all. ...
Review Packet
... 107. What is the minimum amount of uranium called? 108. What is the spontaneous emission of nuclear radiation? 109. Where are protons located? 110. What is the charge of a beta particle? 111. What is a positively charged electron? 112. The measure of which a radioactive substance loses half of its r ...
... 107. What is the minimum amount of uranium called? 108. What is the spontaneous emission of nuclear radiation? 109. Where are protons located? 110. What is the charge of a beta particle? 111. What is a positively charged electron? 112. The measure of which a radioactive substance loses half of its r ...
Final Exam review semester 1
... In science lab, your teacher gives you two small pieces of matter and tells you that one piece is a metal and one is a nonmetal. Without changing the size or shape of the pieces, how could you test them to determine which is the metal? ...
... In science lab, your teacher gives you two small pieces of matter and tells you that one piece is a metal and one is a nonmetal. Without changing the size or shape of the pieces, how could you test them to determine which is the metal? ...
Unit 6 Jeopardy review - Fort Thomas Independent Schools
... Amount of energy needed to start a ...
... Amount of energy needed to start a ...
Answers for Review Questions Exam 3
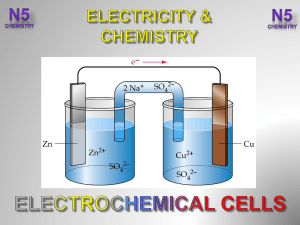
... 9. Electrolysis is the use of an electric current to bring about a chemical change. Reduction and oxidation both occur at the same place as in a galvanic cell, but they have different polarities, - and + respectively. It differs from a galvanic cell in that it is in the opposite direction of a galva ...
... 9. Electrolysis is the use of an electric current to bring about a chemical change. Reduction and oxidation both occur at the same place as in a galvanic cell, but they have different polarities, - and + respectively. It differs from a galvanic cell in that it is in the opposite direction of a galva ...
Answers for Review Questions Exam 3
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
I PUC Chemistry Mock Paper
... 6. Name a compound in which oxidation number of oxygen is +1. 7. Which alkali metal is strongest reducing agent? 8. Name the catalyst used in Friedel Craft’s reaction. 9. Identify the functional group present in CH3CH2COCH3 10. Draw the structure of the trans isomer of But-2-ene. PART – B II. Answer ...
... 6. Name a compound in which oxidation number of oxygen is +1. 7. Which alkali metal is strongest reducing agent? 8. Name the catalyst used in Friedel Craft’s reaction. 9. Identify the functional group present in CH3CH2COCH3 10. Draw the structure of the trans isomer of But-2-ene. PART – B II. Answer ...
South Pasadena • AP Chemistry
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
... 11. When H2SO4 and Ba(OH)2 are reacted in a double replacement reaction, one of the products of the reaction is… a) H2 d) BaH2 b) H2O e) SO2 c) BaS 12. In the double replacement reaction between the weak acid, HC2H3O2 and strong base, NaOH, which ion(s) are spectator ions? a) Na+, C2H3O2– d) H+, C2 ...
Exam practice answers
... Heat loss is the most significant error , which could be improved by lagging the apparatus. Incomplete combustion — CO or C may have been formed , which could be reduced by increasing the supply of oxygen to ensure that CO2 is always produced . (There are 2 marks for the errors and 1 mark for either ...
... Heat loss is the most significant error , which could be improved by lagging the apparatus. Incomplete combustion — CO or C may have been formed , which could be reduced by increasing the supply of oxygen to ensure that CO2 is always produced . (There are 2 marks for the errors and 1 mark for either ...
Conservation of Energy in chemical reactions, Hess`s Law
... happening under standard conditions.) ...
... happening under standard conditions.) ...
Acids and Bases
... • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
... • So NaCl in water dissociates into Na+ and Cl• So H3PO4 dissociates into 3H+ and PO4-3 • Remembers ionic compounds are formed by metals and nonmetals or by metals and polyatomic ions ...
USNCO 2004 National
... 35. A solution is 0. 1 0 M in Ag+, Ca2+, Mg*, and A13+ ions. Which compound will precipitate at the lowest [PO43 ] when a solution of Na3PO4 is added? (A) Ag 3 P0 4 (K a p =lxia 1 6 ) (B) Ca 3 (P0 4 ) 2 (K s p =lxia 3 3 ) (C) Mg 3 (P0 4 ) 2 (K^lxlO- 24 ) ...
... 35. A solution is 0. 1 0 M in Ag+, Ca2+, Mg*, and A13+ ions. Which compound will precipitate at the lowest [PO43 ] when a solution of Na3PO4 is added? (A) Ag 3 P0 4 (K a p =lxia 1 6 ) (B) Ca 3 (P0 4 ) 2 (K s p =lxia 3 3 ) (C) Mg 3 (P0 4 ) 2 (K^lxlO- 24 ) ...
Document
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. ...
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.