Pictures and Graphs
... Conductivity can sometimes be used in place of pH to monitor a reaction. Which of the graphs below best represents the change in conductivity for the following reaction as it is titrated past the equivalence point: HINT: WRITE NET IONIC REACTIONS FOR POINTS THROUGHOUT THE TITRATION. JUSTIFY YOUR CHO ...
... Conductivity can sometimes be used in place of pH to monitor a reaction. Which of the graphs below best represents the change in conductivity for the following reaction as it is titrated past the equivalence point: HINT: WRITE NET IONIC REACTIONS FOR POINTS THROUGHOUT THE TITRATION. JUSTIFY YOUR CHO ...
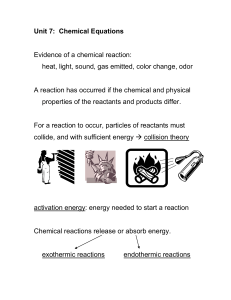
Chemistry: Nuclear Reactions Guided Inquiry + n → + + 3 n +
... have 4 hydrogen atoms and 2 oxygen atoms. Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable ...
... have 4 hydrogen atoms and 2 oxygen atoms. Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable ...
E:\My Documents\sch3u\SCH3Ureview.wpd
... c) Explain why all the atoms in this family form stable ions with this charge. 13) The Alkali Metals are a very reactive family of metals. a) Explain what happens to these atoms when they react with an atom of Chlorine. b) Why do all atoms in this family behave in this manner with Chlorine? c) Potas ...
... c) Explain why all the atoms in this family form stable ions with this charge. 13) The Alkali Metals are a very reactive family of metals. a) Explain what happens to these atoms when they react with an atom of Chlorine. b) Why do all atoms in this family behave in this manner with Chlorine? c) Potas ...
Journal - neutralization
... Grade 10 Academic Science (SNC 2D1) Chemistry - Journal Assignment The Uses of Neutralization Introduction ...
... Grade 10 Academic Science (SNC 2D1) Chemistry - Journal Assignment The Uses of Neutralization Introduction ...
Chapter 12 Review “Stoichiometry”
... Which of the following is NOT true about “yield”? a) the value of actual yield must be known to calculate percent yield, or b) the actual yield may be different from the theoretical yield ...
... Which of the following is NOT true about “yield”? a) the value of actual yield must be known to calculate percent yield, or b) the actual yield may be different from the theoretical yield ...
BONUS: Which line in the above graph represents G for the reaction
... the addition of what ion would effectively increase the S2– concentration? (A) ...
... the addition of what ion would effectively increase the S2– concentration? (A) ...
Describing Chemical Reactions
... Chemical reaction: the process by which one or more substances are changed into one or more new substances Represented by chemical equations Chemical equation: a shorthand expression that represents a chemical reaction Shows the relative amount of each substance taking place in a chemical re ...
... Chemical reaction: the process by which one or more substances are changed into one or more new substances Represented by chemical equations Chemical equation: a shorthand expression that represents a chemical reaction Shows the relative amount of each substance taking place in a chemical re ...
Chemical Equations and Reactions
... acidic solutions to replace the hydrogen in the acid. The products are a metal compound (a salt) and hydrogen gas. ...
... acidic solutions to replace the hydrogen in the acid. The products are a metal compound (a salt) and hydrogen gas. ...
www.studyguide.pk
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
chemeqohnotes18f2005
... Examples: enzymes catalyze biochemical reactions catalytic converters convert CO into CO2 ...
... Examples: enzymes catalyze biochemical reactions catalytic converters convert CO into CO2 ...
reactions taking place within cells
... (8) • Shown as standard value. Experiment not conducted under ‘standard’ conditions • Too many d.ps/significant figures. Accuracy of apparatus doesn’t warrant • Not shown as negative. Exothermic reaction ...
... (8) • Shown as standard value. Experiment not conducted under ‘standard’ conditions • Too many d.ps/significant figures. Accuracy of apparatus doesn’t warrant • Not shown as negative. Exothermic reaction ...
CHM1 Exam 16 Name 2222222222222222222222222222 Multiple
... 19. Based on the following reaction 2 N2 (g) + 5 O2 (g) 2 N2O5 (g) How many gram of N2O5 could theoretically be formed by reacting 10.0 g of elemental nitrogen with 12.0 g of elemental oxygen? (1) 27.1 g (2) 11.3 g ...
... 19. Based on the following reaction 2 N2 (g) + 5 O2 (g) 2 N2O5 (g) How many gram of N2O5 could theoretically be formed by reacting 10.0 g of elemental nitrogen with 12.0 g of elemental oxygen? (1) 27.1 g (2) 11.3 g ...
program
... make a connection between bond types, lattice type, and the properties of a substance: • melting point and boiling point; • hardness and brittleness; • absence or presence of electrical conductivity in the solid, liquid, and/or dissolved ...
... make a connection between bond types, lattice type, and the properties of a substance: • melting point and boiling point; • hardness and brittleness; • absence or presence of electrical conductivity in the solid, liquid, and/or dissolved ...
Thermochemistry - Ars
... occurs at a higher temperature by finding reactions that occur at lower temperatures that add to give the correct overall equation. Standard Enthalpies of Formation Another way to determine the enthalpy of reaction is to utilize standard enthalpies of formation, ∆ Hf° . These are tabulated values (s ...
... occurs at a higher temperature by finding reactions that occur at lower temperatures that add to give the correct overall equation. Standard Enthalpies of Formation Another way to determine the enthalpy of reaction is to utilize standard enthalpies of formation, ∆ Hf° . These are tabulated values (s ...
Learning Guide – Poisons (I)
... Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how many units of each compound are neede ...
... Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how many units of each compound are neede ...
PowerPoint Lectures - Northwest ISD Moodle
... When a carbonate or bicarbonate reacts with an acid, the products are a salt, carbon dioxide, and water. ...
... When a carbonate or bicarbonate reacts with an acid, the products are a salt, carbon dioxide, and water. ...
CHEMISTRY A
... Explain why the atom economy of this cyclohexene preparation is higher than that from cyclohexanol in (c). ...
... Explain why the atom economy of this cyclohexene preparation is higher than that from cyclohexanol in (c). ...
9.1 Electron Transfer Reactions
... oxidation-reduction reactions according to certain rules • An atom’s oxidation number is the positive or negative charge on the atom if the electron pairs in a covalent bond belong only to the more electronegative atom ...
... oxidation-reduction reactions according to certain rules • An atom’s oxidation number is the positive or negative charge on the atom if the electron pairs in a covalent bond belong only to the more electronegative atom ...
GENERAL CHEMISTRY REVIEW
... Binary Ionic Compounds, where the metal ion has variable oxidation state (Transition elements) 1. the oxidation state on the metal ion is specified by Roman Numeral in brackets 2. monoatomic anions are named as before For example, CuCl and CuCl2 are named as copper (I) chloride and copper (II) chlor ...
... Binary Ionic Compounds, where the metal ion has variable oxidation state (Transition elements) 1. the oxidation state on the metal ion is specified by Roman Numeral in brackets 2. monoatomic anions are named as before For example, CuCl and CuCl2 are named as copper (I) chloride and copper (II) chlor ...
Spring 2014
... that the work of others in this class has, to the best of my knowledge, been honest as well. Signed __________________________________________________________________________ If you feel you can’t sign this, contact the instructor (e-mail or in person) (2 pts each) Multiple choice - circle the corre ...
... that the work of others in this class has, to the best of my knowledge, been honest as well. Signed __________________________________________________________________________ If you feel you can’t sign this, contact the instructor (e-mail or in person) (2 pts each) Multiple choice - circle the corre ...
Chemistry 2nd Semester Final Review
... 2. A gas has a volume of 1.49 L at a temperature of 34.75 °C. What would the volume be at 78.41 °C? (pressure & amt. of gas constant) 3. What volume is occupied by 8.47 g of hydrogen gas at 84.7 °C and 1.04 atm? 4. What volume is occupied by 56.75 g of oxygen gas at STP? 5. If 15.71 g of oxygen gas ...
... 2. A gas has a volume of 1.49 L at a temperature of 34.75 °C. What would the volume be at 78.41 °C? (pressure & amt. of gas constant) 3. What volume is occupied by 8.47 g of hydrogen gas at 84.7 °C and 1.04 atm? 4. What volume is occupied by 56.75 g of oxygen gas at STP? 5. If 15.71 g of oxygen gas ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.