Thermochemistry
... Overview: We will learn and implement some familiar math to find ΔH for reactions of interest – this is Hess’ Law. Important fact: Enthalpy is an example of a State Function (see appendix / slide). This fact makes the math possible. Enthalpy as a state function: ‘It doesn’t matter how you get there ...
... Overview: We will learn and implement some familiar math to find ΔH for reactions of interest – this is Hess’ Law. Important fact: Enthalpy is an example of a State Function (see appendix / slide). This fact makes the math possible. Enthalpy as a state function: ‘It doesn’t matter how you get there ...
Practice exam - Dynamic Science
... 21) Pure ethanol can be produced from wine by a process best known as: a) b) c) d) ...
... 21) Pure ethanol can be produced from wine by a process best known as: a) b) c) d) ...
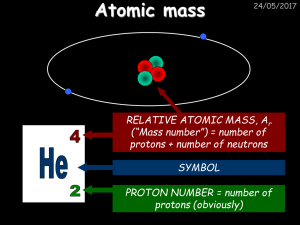
Atomic mass - drseemaljelani
... reaction, it is not always possible to obtain the calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the ...
... reaction, it is not always possible to obtain the calculated amount of product. Because: • The reaction may not totally finish – it may be reversible • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the ...
File
... Catalyst: something that speeds up a chemical reaction Enzyme: proteins that speed up/help jump start reactions in an organism Therefore enzymes are catalysts because they speed up biochemical reactions • We need enzymes for every process that happens in our bodies! ...
... Catalyst: something that speeds up a chemical reaction Enzyme: proteins that speed up/help jump start reactions in an organism Therefore enzymes are catalysts because they speed up biochemical reactions • We need enzymes for every process that happens in our bodies! ...
AP Chemistry: Aqueous Reactions and Solution Stoichiometry
... 2In this reaction the neutral O2 has gained electrons from the Ca to become O in CaO. 2We say O2 has been reduced to O . In all reduction-oxidation (redox) reactions, one species is reduced at the same time as another is oxidized. Oxidation Numbers Electrons are not explicitly shown in chemical equa ...
... 2In this reaction the neutral O2 has gained electrons from the Ca to become O in CaO. 2We say O2 has been reduced to O . In all reduction-oxidation (redox) reactions, one species is reduced at the same time as another is oxidized. Oxidation Numbers Electrons are not explicitly shown in chemical equa ...
1 - Montville.net
... AP Chemistry can be considered as the second year of a two year program. The topics covered at the beginning of the year are mostly review from first year chemistry so we will move very quickly through this material. The purpose of this assignment is to review some of the material you learned last y ...
... AP Chemistry can be considered as the second year of a two year program. The topics covered at the beginning of the year are mostly review from first year chemistry so we will move very quickly through this material. The purpose of this assignment is to review some of the material you learned last y ...
Chemistry II Exams and Keys 2014 Season
... C. nitric acid D. perchloric acid 6. A bomb calorimeter is calibrated by combusting 1.558 g of benzoic acid (MW = 122.2 g/mol) in the chamber. The temperature of the water is increased by 2.34 K. The enthalpy of combustion of benzoic acid is -3230 kJ/mol. After determining the calorimetric constant, ...
... C. nitric acid D. perchloric acid 6. A bomb calorimeter is calibrated by combusting 1.558 g of benzoic acid (MW = 122.2 g/mol) in the chamber. The temperature of the water is increased by 2.34 K. The enthalpy of combustion of benzoic acid is -3230 kJ/mol. After determining the calorimetric constant, ...
Contents
... example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic devices is produced by converting silicon dioxide (the mineral quartz) to the element silic ...
... example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic devices is produced by converting silicon dioxide (the mineral quartz) to the element silic ...
Microsoft Word
... Covalent Bonding Elements which are neither highly electropositive nor highly electronegative are less likely to form ionic compounds. Consider CH4, methane; carbon and hydrogen have similar electronegativities. It would be inappropriate to describe the bonds between carbon and hydrogen in methane a ...
... Covalent Bonding Elements which are neither highly electropositive nor highly electronegative are less likely to form ionic compounds. Consider CH4, methane; carbon and hydrogen have similar electronegativities. It would be inappropriate to describe the bonds between carbon and hydrogen in methane a ...
CH 4 Notes
... In this reaction the neutral O2 has gained electrons from the Ca to become O2- in CaO. We say O2 has been reduced to O2-. In all reduction-oxidation (redox) reactions, one species is reduced at the same time as another is oxidized. Oxidation Numbers Electrons are not explicitly shown in chem ...
... In this reaction the neutral O2 has gained electrons from the Ca to become O2- in CaO. We say O2 has been reduced to O2-. In all reduction-oxidation (redox) reactions, one species is reduced at the same time as another is oxidized. Oxidation Numbers Electrons are not explicitly shown in chem ...
Gupta 2014 Credit: Google Images for the pictures Chapter 1
... 1. Name cation before anion; one or both may be a complex. (Follow standard nomenclature for noncomplexes.) 2. Within each complex (neutral or ion), name all ligands before the metal. -Name ligands in alphabetical order -If more than one of the same ligand is present, use a numerical prefix: di, tri ...
... 1. Name cation before anion; one or both may be a complex. (Follow standard nomenclature for noncomplexes.) 2. Within each complex (neutral or ion), name all ligands before the metal. -Name ligands in alphabetical order -If more than one of the same ligand is present, use a numerical prefix: di, tri ...
Table of Contents - Free Coursework for GCSE, IGCSE, A Level, IB
... A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high energy end of the spectrum. The lines converge as the shells are getting closer together. Energy levels increase because we get a ...
... A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high energy end of the spectrum. The lines converge as the shells are getting closer together. Energy levels increase because we get a ...
Atomic Theory
... A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high energy end of the spectrum. The lines converge as the shells are getting closer together. Energy levels increase because we get a ...
... A line spectrum is not continuous. Each element has its own characteristic line spectrum. Hydrogen spectrum- it consists of discrete lines that converge towards the high energy end of the spectrum. The lines converge as the shells are getting closer together. Energy levels increase because we get a ...
Old EXAM I - gozips.uakron.edu
... the lighter object. both objects have the same volume. it is impossible to tell without additional information. ...
... the lighter object. both objects have the same volume. it is impossible to tell without additional information. ...
Research on Hydrogenation of FAME to Fatty Alcohols
... velocity increased. Compared with 0.15~0.4h-1 space velocity which calculated according to tradition process of hydrogenolysis of fatty acid methyl ester, supercritical reaction technology was taken, but there was 90% solvent remaining in reaction materials. When the space velocity of mixture made o ...
... velocity increased. Compared with 0.15~0.4h-1 space velocity which calculated according to tradition process of hydrogenolysis of fatty acid methyl ester, supercritical reaction technology was taken, but there was 90% solvent remaining in reaction materials. When the space velocity of mixture made o ...
synthesis-structure relationship in the aqueous ethylene glycol
... unconventional methods for their preparation. In this context, starting with the ’80s, a new preparation method for complexes with glyoxylate anion as ligand was established.15,33 It is well known that, depending on the oxidation agent used and the working conditions applied, polyols can be oxidized ...
... unconventional methods for their preparation. In this context, starting with the ’80s, a new preparation method for complexes with glyoxylate anion as ligand was established.15,33 It is well known that, depending on the oxidation agent used and the working conditions applied, polyols can be oxidized ...
9791/02 UNIVERSITY OF CAMBRIDGE INTERNATIONAL
... Chemists have recently managed to prepare P2 molecules in the solid state, trapped in an organic framework (reported in Nature Chemistry, 2010). The labelled molecular orbital diagram represents the bonding in P2, which has a bond order of 3. ...
... Chemists have recently managed to prepare P2 molecules in the solid state, trapped in an organic framework (reported in Nature Chemistry, 2010). The labelled molecular orbital diagram represents the bonding in P2, which has a bond order of 3. ...
Strecker Degradation Products of Aspartic and Glutamic Acids and
... levels similar to those given above. Propionaldehyde was another product identified but the intermediate α-ketoglutaric acid was not identified. Dicarboxylic and other nonvolatile acids were analysed as methyl esters and the results obtained are summarized in Table 2. As can be seen, fumaric acid ar ...
... levels similar to those given above. Propionaldehyde was another product identified but the intermediate α-ketoglutaric acid was not identified. Dicarboxylic and other nonvolatile acids were analysed as methyl esters and the results obtained are summarized in Table 2. As can be seen, fumaric acid ar ...
2010 `A` Levels Suggested Solutions
... This reaction is unusual as you’ve been taught that alcohols are neutral. But note that the use of conc HCl is to remove the organic impurity that cannot be separate by Step 5. The ONLY organic reactant used is butan-1-ol making it the only possible candidate as the organic impure that the question ...
... This reaction is unusual as you’ve been taught that alcohols are neutral. But note that the use of conc HCl is to remove the organic impurity that cannot be separate by Step 5. The ONLY organic reactant used is butan-1-ol making it the only possible candidate as the organic impure that the question ...
Solution
... completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation. 4. Check that charges and number of atoms are balanced in the net ionic equation. ...
... completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation. 4. Check that charges and number of atoms are balanced in the net ionic equation. ...
Unit 2.7: Periodic Table Group1 Group2 Li Be Na Mg K Ca Rb Sr Cs
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
g - Santa Rosa Junior College
... from the atmosphere, where they occur as the free elements. A few elements occur naturally in their uncombined (native) state. These include S, carbon in coal, and unreactive metals. ...
... from the atmosphere, where they occur as the free elements. A few elements occur naturally in their uncombined (native) state. These include S, carbon in coal, and unreactive metals. ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.