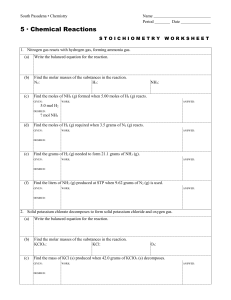
Investigating the kinetic mechanisms of the oxygen
... exists. The formation of Li2O is kinetically limited, and the major discharge product is Li2O2, even at standard potentials more negative than 2.91 V. In theoretical and experimental studies using density functional theory (DFT) and spectroscopic measurements, respectively, Li2O2 production was repo ...
... exists. The formation of Li2O is kinetically limited, and the major discharge product is Li2O2, even at standard potentials more negative than 2.91 V. In theoretical and experimental studies using density functional theory (DFT) and spectroscopic measurements, respectively, Li2O2 production was repo ...
Chapter 13 notes
... water, they yield only : HCl, HBr and HI are 100% ionized in dilute aqueous ...
... water, they yield only : HCl, HBr and HI are 100% ionized in dilute aqueous ...
in-class assignment - hrsbstaff.ednet.ns.ca
... see and hear it as titanium dioxide. So far the ions I've shown were of single atoms, for example, Na+, K+, Ti4+, Cl-, and O2-. However, a large number of compounds have ions that are made from two or more elements. They call them polyatomic ions. "Poly" meaning "many" and "atomic" referring to atom ...
... see and hear it as titanium dioxide. So far the ions I've shown were of single atoms, for example, Na+, K+, Ti4+, Cl-, and O2-. However, a large number of compounds have ions that are made from two or more elements. They call them polyatomic ions. "Poly" meaning "many" and "atomic" referring to atom ...
AP Semestar Exam REVIEW
... d. enthalpy is a state property. e. in an endothermic process heat flows from the surroundings into the system. ...
... d. enthalpy is a state property. e. in an endothermic process heat flows from the surroundings into the system. ...
Chemical Formulas and Equations
... • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
... • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a ...
Chemistry Final Test 1999-2000 - Nashoba Valley Technical High
... 39) The illustration below shows two hydrogen molecules reacting with an oxygen molecule to form two water molecules. What type of bonding is formed in the water molecules? ...
... 39) The illustration below shows two hydrogen molecules reacting with an oxygen molecule to form two water molecules. What type of bonding is formed in the water molecules? ...
6-1 Endothermic and Exothermic Reactions
... someone take note of the initial temperature. SLOWLY add ACID TO WATER. Take temperature again. Typical temperature change will be from around 22o C to 93o C. The equation for this reaction is: H2O (l) + H2SO4 (aq) = H2O (l) + H2SO4 (aq) (diluted) The amount of heat in Joules can be calculated as fo ...
... someone take note of the initial temperature. SLOWLY add ACID TO WATER. Take temperature again. Typical temperature change will be from around 22o C to 93o C. The equation for this reaction is: H2O (l) + H2SO4 (aq) = H2O (l) + H2SO4 (aq) (diluted) The amount of heat in Joules can be calculated as fo ...
Balanced Chemical Reaction Equations
... reaction equations. Would you like to hear about them? The three friends loudly shout in unison, yes! Dr Dave: A complete equation specifies the state of the reactants and products with a symbol: (s) for solid, (g) for gas, (l) for liquid, and (aq) for dissolved in water. So the copper/silver reac ...
... reaction equations. Would you like to hear about them? The three friends loudly shout in unison, yes! Dr Dave: A complete equation specifies the state of the reactants and products with a symbol: (s) for solid, (g) for gas, (l) for liquid, and (aq) for dissolved in water. So the copper/silver reac ...
Contents
... product, some waste chemicals are produced. These not only contribute to pollution problems but they are also costly for the chemical company. For this reason, chemists are devising new chemical reactions which will enable our products to be manufactured in a more chemicallyefficient process with le ...
... product, some waste chemicals are produced. These not only contribute to pollution problems but they are also costly for the chemical company. For this reason, chemists are devising new chemical reactions which will enable our products to be manufactured in a more chemicallyefficient process with le ...
PP - Columbia University
... • So when calulating Go, instead of writing in “55” when water participates in a reaction (e.g., a hydrolysis) we write “1.” • This is not cheating; we are in charge of what is a “standard” condition, and we all agree to this: 55 M H20 is unit (“1”) concentration for the purpose of defining Go. ...
... • So when calulating Go, instead of writing in “55” when water participates in a reaction (e.g., a hydrolysis) we write “1.” • This is not cheating; we are in charge of what is a “standard” condition, and we all agree to this: 55 M H20 is unit (“1”) concentration for the purpose of defining Go. ...
August 2010 Regents Exam part 1
... 19 Petroleum can be separated by distillation because the hydrocarbons in petroleum are (1) elements with identical boiling points (2) elements with different boiling points (3) compounds with identical boiling points (4) compounds with different boiling points (fractional distillation or cracking, ...
... 19 Petroleum can be separated by distillation because the hydrocarbons in petroleum are (1) elements with identical boiling points (2) elements with different boiling points (3) compounds with identical boiling points (4) compounds with different boiling points (fractional distillation or cracking, ...
Unit 2.7: Periodic Table Group1 Group2 Li Be Na Mg K Ca Rb Sr Cs
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
... Group 2 metals have higher melting temperature than group1 metals in the same period. This is because each atom loses two electrons to form the metallic bond, which is therefore stronger than metallic bond in group 1 metal and also metallic radius of group2 elements is smaller than group1 elements i ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... *e. There are currently 118 known ______________ and ___ are found in nature, while the others are ________________________ (man-made), but we only use between 30-40 elements daily *1. The discovery of all the ___________________ to date has taken THOUSANDS of years *2. In ancient times, it was beli ...
... *e. There are currently 118 known ______________ and ___ are found in nature, while the others are ________________________ (man-made), but we only use between 30-40 elements daily *1. The discovery of all the ___________________ to date has taken THOUSANDS of years *2. In ancient times, it was beli ...
Honors-Final-Review-2014
... a. A solution that keeps a constant neutral pH when small amounts of acid or base are added b. Solution of known concentration c. Acid contains one H d. Acid contains three or more H’s e. The point at which the indicator changes color f. Any substance that accepts a proton g. Any substance that dona ...
... a. A solution that keeps a constant neutral pH when small amounts of acid or base are added b. Solution of known concentration c. Acid contains one H d. Acid contains three or more H’s e. The point at which the indicator changes color f. Any substance that accepts a proton g. Any substance that dona ...
Introduction_to_Chemical_Reactions_2011
... • The heat energy that moves between the system and surroundings during chemical reactions is basically the energy that is used to break bonds and the energy that is released when bonds form. (i.e. bond energy) • The energy change that accompanies any chemical reaction is called the enthalpy (heat) ...
... • The heat energy that moves between the system and surroundings during chemical reactions is basically the energy that is used to break bonds and the energy that is released when bonds form. (i.e. bond energy) • The energy change that accompanies any chemical reaction is called the enthalpy (heat) ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... Cl2 + 2 KBr → 2 KCl + Br2 Zn + CuSO4 → ZnSO4 + Cu The molecular equations can be converted to net-ionic form by applying solubility rules. Note that the activity of a given element is most easily predicted with an “activity series” or a standard reduction potential table. Since there are no “no reac ...
... Cl2 + 2 KBr → 2 KCl + Br2 Zn + CuSO4 → ZnSO4 + Cu The molecular equations can be converted to net-ionic form by applying solubility rules. Note that the activity of a given element is most easily predicted with an “activity series” or a standard reduction potential table. Since there are no “no reac ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.