LN_atoms_etc
... Modern View of Atomic Structure Experiments by Thomson and Millikan confirmed the existence of electrons as the negatively charged particles within an atom. Electrons have a charge of e = 1.6021773 10–19 C and a mass of 9.109390 10–31 kg. Later experiments by Rutherford determined that at the ce ...
... Modern View of Atomic Structure Experiments by Thomson and Millikan confirmed the existence of electrons as the negatively charged particles within an atom. Electrons have a charge of e = 1.6021773 10–19 C and a mass of 9.109390 10–31 kg. Later experiments by Rutherford determined that at the ce ...
spring semester review
... e) S4O6260. Which substance is the oxidizing agent in the following reaction: Pb + PbO2 + 2H2SO4 --> 2PbSO4 + 2H20 a) Pb b) H2SO4 c) PbO2 d) PbSO4 e) H2O 61. What is the oxidation number of each sulfur atom in manganese (II) sulfate? a) -2 b) +3 c) +6 d) -1 e) 0 62. The electrode at which oxidation ...
... e) S4O6260. Which substance is the oxidizing agent in the following reaction: Pb + PbO2 + 2H2SO4 --> 2PbSO4 + 2H20 a) Pb b) H2SO4 c) PbO2 d) PbSO4 e) H2O 61. What is the oxidation number of each sulfur atom in manganese (II) sulfate? a) -2 b) +3 c) +6 d) -1 e) 0 62. The electrode at which oxidation ...
AP Chemistry Summer Assignment
... order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. With the ready access to hundreds of websites either in your home or at the local l ...
... order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. There is a multitude of tremendous chemistry resources are available via the Internet. With the ready access to hundreds of websites either in your home or at the local l ...
File
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
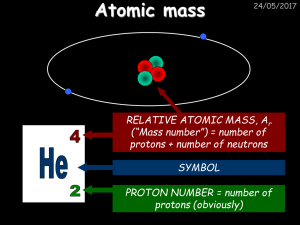
Atomic mass - drseemaljelani
... • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
... • Some of the product may be lost when it is separated from the reaction mixture – filtered • Some of the reactants may react in different ways to the expected reaction ...
Science Olympiad Circuit Lab
... Electric Potential Difference (∆V) – the electric potential energy per unit charge. Volt (V) – SI unit of Electric potential difference (1V = 1J/coul). Electromotive Force (EMF) – any device which can establish an electric potential difference across a circuit, e.g. battery, generator, alternator, p ...
... Electric Potential Difference (∆V) – the electric potential energy per unit charge. Volt (V) – SI unit of Electric potential difference (1V = 1J/coul). Electromotive Force (EMF) – any device which can establish an electric potential difference across a circuit, e.g. battery, generator, alternator, p ...
Unit: Corrosion Science Important Questions with Hints
... [Zn(NH3)4]2+ . This results in dissolution of brass leading to formation of fissures, which propagate and lead to development of cracks. 17. What do you mean by the terms galvanization and tinning? Hints: Coating of zinc on iron by hot dipping is called galvanization and coating of tin on iron is ca ...
... [Zn(NH3)4]2+ . This results in dissolution of brass leading to formation of fissures, which propagate and lead to development of cracks. 17. What do you mean by the terms galvanization and tinning? Hints: Coating of zinc on iron by hot dipping is called galvanization and coating of tin on iron is ca ...
Which notation represents an atom of sodium
... Base your answers to questions 1 and 2 on the information below. Ozone gas, O3, can be used to kill adult insects in storage bins for grain without damaging the grain. The ozone is roduced from oxygen gas, O2, in portable ozone generators located near the storage bins. The concentrations of ozone us ...
... Base your answers to questions 1 and 2 on the information below. Ozone gas, O3, can be used to kill adult insects in storage bins for grain without damaging the grain. The ozone is roduced from oxygen gas, O2, in portable ozone generators located near the storage bins. The concentrations of ozone us ...
Students know
... C. Gas molecules move faster when heated and this causes them to move out of the solution so they don’t dissolve. D. Gas molecules are lower in energy than water, therefore when the gas molecules are heated they have an increase in their total enthalpy of dissolution which requires an increase in th ...
... C. Gas molecules move faster when heated and this causes them to move out of the solution so they don’t dissolve. D. Gas molecules are lower in energy than water, therefore when the gas molecules are heated they have an increase in their total enthalpy of dissolution which requires an increase in th ...
2011 Assessment Report Chemistry 2
... PbO2(s) + 4H+(aq) + SO42-(aq) + 2ePbSO4(s) + 2H2O(l) E = +1.69 V PbSO4(s) + 2ePb(s) + SO42-(aq) E = 0.36 V it can be deduced that the reduction of strongest oxidant, PbO2(s), is accompanied by a decrease in [H+] and so the pH increases. During recharging, electrical energy is converted into chemi ...
... PbO2(s) + 4H+(aq) + SO42-(aq) + 2ePbSO4(s) + 2H2O(l) E = +1.69 V PbSO4(s) + 2ePb(s) + SO42-(aq) E = 0.36 V it can be deduced that the reduction of strongest oxidant, PbO2(s), is accompanied by a decrease in [H+] and so the pH increases. During recharging, electrical energy is converted into chemi ...
Higher Chemistry summary 3a
... Therefore, for every 0.5 moles of methane 1 mole of oxygen would be required. Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen wil ...
... Therefore, for every 0.5 moles of methane 1 mole of oxygen would be required. Looking at the quantities of reactants from step 1 there is not enough oxygen to allow all of the methane to react therefore some methane will be left over at the end. The methane is said to be in excess and the oxygen wil ...
Chemistry I Exams and Keys 2014 Season
... Among the alkali metals, cesium reacts more rapidly with water than sodium. To what ...
... Among the alkali metals, cesium reacts more rapidly with water than sodium. To what ...
A roller coaster ride is a thrilling experience which involves a wealth
... Entropy is a measure of the disorder of a system the more disordered the higher the entropy. Systems gain stability and entropy by being more disordered (randomized) so lets look at some physical examples Most disordered Gases, ions in solution, liquids. Solids Both enthalpy and entropy combined hel ...
... Entropy is a measure of the disorder of a system the more disordered the higher the entropy. Systems gain stability and entropy by being more disordered (randomized) so lets look at some physical examples Most disordered Gases, ions in solution, liquids. Solids Both enthalpy and entropy combined hel ...
APS Practice Final 2011
... ____ 55. You have 85.5 g of fluorine, which has a molar mass of approximately 19 g/mol. How many moles of fluorine do you have? a. 4.5 mol c. 45 mol b. 19 mol d. 85 mol ____ 56. The forces that hold different atoms or ions together are a. electric currents. c. physical bonds. b. chemical bonds. d. n ...
... ____ 55. You have 85.5 g of fluorine, which has a molar mass of approximately 19 g/mol. How many moles of fluorine do you have? a. 4.5 mol c. 45 mol b. 19 mol d. 85 mol ____ 56. The forces that hold different atoms or ions together are a. electric currents. c. physical bonds. b. chemical bonds. d. n ...
C:\Users\mrh70950\Documents\My Files\WordPerfect
... 1. elimination of 2 HX from geminal, 1,1-dihaloalkanes: double dehydrohalogenation using very strong bases a. E2 twice is usually mechanism b. must use strong base like NaNH2 c. anti-elimination stereochemistry of E2 is followed d. an intermediate haloalkene is generated; this alkene is the major pr ...
... 1. elimination of 2 HX from geminal, 1,1-dihaloalkanes: double dehydrohalogenation using very strong bases a. E2 twice is usually mechanism b. must use strong base like NaNH2 c. anti-elimination stereochemistry of E2 is followed d. an intermediate haloalkene is generated; this alkene is the major pr ...
04-01VoltageandElectricField
... E = -ΔV/Δx, ΔV = 1.50 V,, E = 13 V/m Δx = 0.1154 m = 0.12 m = 12 cm ...
... E = -ΔV/Δx, ΔV = 1.50 V,, E = 13 V/m Δx = 0.1154 m = 0.12 m = 12 cm ...
unit 7 h chem notes - chemical equations
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
... II. Sometimes it is necessary to abbreviate the “phase” of the substance to the lower right of the substance. Some abbreviations are: s = solid, l= liquid, g ( )= gas, aq= aqueous, ppt ( )= precipitate. III Write equations using correct formulas of diatomic molecules, then Balance the equation for e ...
Unit 4
... 4.6 Classification of Chemical Reactions There is no comprehensive classification scheme that would accommodate all known chemical reactions. One approach is to classify reactions into four types: combination, decomposition, single replacement and double replacement reactions. I) Combination Reacti ...
... 4.6 Classification of Chemical Reactions There is no comprehensive classification scheme that would accommodate all known chemical reactions. One approach is to classify reactions into four types: combination, decomposition, single replacement and double replacement reactions. I) Combination Reacti ...
LIQUIDS
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
... After element 20 the electron arrangement becomes more complicated, but it is always true that elements in Group 1 have one electron in their outer shell, so we can say that Rb, Cs and Fr will all have one electron in their outer shell. Therefore elements in Group 3 always have three electrons in th ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.