! !! ! n nn N P =
... A. Energy can never be created or destroyed but it can be changed from one form to another. B. Two bodies in thermal contact are at thermal equilibrium with each other if the two bodies are at the same absolute temperature. C. Any process carried out in several steps, the overall ∆H is equal to the ...
... A. Energy can never be created or destroyed but it can be changed from one form to another. B. Two bodies in thermal contact are at thermal equilibrium with each other if the two bodies are at the same absolute temperature. C. Any process carried out in several steps, the overall ∆H is equal to the ...
Practice Problem Set #6
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
... 1. Write balanced chemical equations for the reaction of hydrogen gas with oxygen, chlorine, and nitrogen. 2. Write a balanced chemical equation for the preparation of H2 (and CO) by the reaction of CH4 and water. Using a table of thermodynamic data, calculate ∆H°, ∆G°, and ∆S° for this reaction. ...
Lab #4: Chemical Reactions
... Lab #4: Chemical Reactions Many chemical reactions can be placed into one of two categories: oxidation-reduction reactions and double replacement reactions. Oxidation-reduction reactions are ones in which electrons are transferred from one species to another. There are four types of oxidation-reduct ...
... Lab #4: Chemical Reactions Many chemical reactions can be placed into one of two categories: oxidation-reduction reactions and double replacement reactions. Oxidation-reduction reactions are ones in which electrons are transferred from one species to another. There are four types of oxidation-reduct ...
Aqueous Solutions
... •Oxidation is an increase in the oxidation number. (氧化數增加) – Corresponds to the loss of electrons. 失去電子 •Reduction is a decrease in the oxidation number. (氧化數減少) – Good mnemonic – reduction reduces the oxidation number. – Corresponds to the gain of electrons 得到電子 ...
... •Oxidation is an increase in the oxidation number. (氧化數增加) – Corresponds to the loss of electrons. 失去電子 •Reduction is a decrease in the oxidation number. (氧化數減少) – Good mnemonic – reduction reduces the oxidation number. – Corresponds to the gain of electrons 得到電子 ...
Redox reactions - SALEM-Immanuel Lutheran College
... 7/2KMnO4(s) + C3H5(OH)3(l)K2CO3(s)+7/4Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)K2CO3(s)+7Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+5CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+5CO2 ...
... 7/2KMnO4(s) + C3H5(OH)3(l)K2CO3(s)+7/4Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)K2CO3(s)+7Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+5CO2(g)+ H2O(l) 14KMnO4(s) + 4C3H5(OH)3(l)7K2CO3(s)+7Mn2O3(s)+5CO2 ...
Chapter 12 Review “Stoichiometry”
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
Chapter 12 Review “Stoichiometry”
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
Chapter 12 Review “Stoichiometry”
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
... 0.1 mol of Ca reacts with 880 g water, 2.24 L of hydrogen gas forms (at STP). How would the amount of hydrogen produced change if the volume of water was decreased to 440 mL (440 g)? When two substances react to form products, the reactant which is used up is called the ____. ...
p-Block Elements, Part 1
... e.g. Li2O = 2Li+ O2− Peroxide Ion ⇒ O22− = −O – O− e.g. Na2O2 = 2 Na+ −O – O − Also, H2O2 (hydrogen peroxide) Superoxide Ion ⇒ O2− e.g. KO2 = K+ O2− Can have positive oxidation states in combination with fluorine + 2 in OF2 ...
... e.g. Li2O = 2Li+ O2− Peroxide Ion ⇒ O22− = −O – O− e.g. Na2O2 = 2 Na+ −O – O − Also, H2O2 (hydrogen peroxide) Superoxide Ion ⇒ O2− e.g. KO2 = K+ O2− Can have positive oxidation states in combination with fluorine + 2 in OF2 ...
Welcome to AP Chemistry! AP Chemistry is
... nickel (II) carbonate copper (II) hydroxide tin (IV) sulfate ...
... nickel (II) carbonate copper (II) hydroxide tin (IV) sulfate ...
biology biology - Napa Valley College
... A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons A triple covalent bond, or triple bond, is the sharing of three pairs of valence electrons ...
... A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons A triple covalent bond, or triple bond, is the sharing of three pairs of valence electrons ...
KEY Final Exam Review - Iowa State University
... NO3¯ ---> NO 2) balance each half-reaction: 8H2S ---> S8 + 16H+ + 16e¯ 3e¯ + 4H+ + NO3¯ ---> NO + 2H2O 3) Make the number of electrons equal: 24H2S ---> 3S8 + 48H+ + 48e¯ <--- multiplied by a factor of 3 48e¯ + 64H+ + 16NO3¯ ---> 16NO + 32H2O <--- multiplied by a factor of 16 Note that 16 and 3 have ...
... NO3¯ ---> NO 2) balance each half-reaction: 8H2S ---> S8 + 16H+ + 16e¯ 3e¯ + 4H+ + NO3¯ ---> NO + 2H2O 3) Make the number of electrons equal: 24H2S ---> 3S8 + 48H+ + 48e¯ <--- multiplied by a factor of 3 48e¯ + 64H+ + 16NO3¯ ---> 16NO + 32H2O <--- multiplied by a factor of 16 Note that 16 and 3 have ...
Thermo Practice Test
... 26. T - F For the process in #25, we would expect S to decrease with increasing pressure. 27. T - F For the decomposition of water to the elements at standard conditions, G= +56.7 kcal. This means that at least 56.7 kcal of work (energy) has to be supplied to make this reaction go. ...
... 26. T - F For the process in #25, we would expect S to decrease with increasing pressure. 27. T - F For the decomposition of water to the elements at standard conditions, G= +56.7 kcal. This means that at least 56.7 kcal of work (energy) has to be supplied to make this reaction go. ...
PowerPoint - Balancing Equations
... Balancing equations: MgO • The law of conservation of mass states that matter can neither be created or destroyed • Thus, atoms are neither created or destroyed, only rearranged in a chemical reaction • Thus, the number of a particular atom is the same on both sides of a chemical equation • Example ...
... Balancing equations: MgO • The law of conservation of mass states that matter can neither be created or destroyed • Thus, atoms are neither created or destroyed, only rearranged in a chemical reaction • Thus, the number of a particular atom is the same on both sides of a chemical equation • Example ...
PS_CHEM7_ch4 - WordPress.com
... • d) Ethylene glycol (HOCH2CH2OH) molecules contain polar O–H bonds, similar to water, so it would be expected to be soluble. ...
... • d) Ethylene glycol (HOCH2CH2OH) molecules contain polar O–H bonds, similar to water, so it would be expected to be soluble. ...
10562_2013_1023_MOESM1_ESM
... The Computational Hydrogen Electrode Model Free energy diagrams are constructed using the Computational Hydrogen Electrode (CHE) model [6, 7]. The method is identical to the method used in previous work on CO2 reduction [7], but given here for completeness. To calculate the reaction free energy of a ...
... The Computational Hydrogen Electrode Model Free energy diagrams are constructed using the Computational Hydrogen Electrode (CHE) model [6, 7]. The method is identical to the method used in previous work on CO2 reduction [7], but given here for completeness. To calculate the reaction free energy of a ...
lesson 5
... 9. How many minus charges does each fluorine atom gain? 10. How many minus charges does each fluorine atom now have? ...
... 9. How many minus charges does each fluorine atom gain? 10. How many minus charges does each fluorine atom now have? ...
Electrochemistry
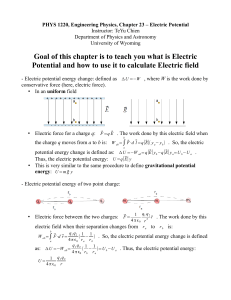
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.