General Chemistry Review Problems
... CO2(g) + 2LiOH(s) Li2CO3(s) + H2O(l) a. If 1.20x1024 molecules of CO2 is exhaled, the average amount exhaled by a person each day, how much (in grams) Li2CO3 is produced? b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to co ...
... CO2(g) + 2LiOH(s) Li2CO3(s) + H2O(l) a. If 1.20x1024 molecules of CO2 is exhaled, the average amount exhaled by a person each day, how much (in grams) Li2CO3 is produced? b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to co ...
Summer Resources - mvhs
... When a compound containing C,H and O undergoes combustion, it forms CO2 and H2O. Then from the mass of CO2 and H2O, we can calculate the mass of C and Hand then find the mass of O by subtracting the sum of masses of C and H from total g present of that substance. From the mass of C,H and O, we can c ...
... When a compound containing C,H and O undergoes combustion, it forms CO2 and H2O. Then from the mass of CO2 and H2O, we can calculate the mass of C and Hand then find the mass of O by subtracting the sum of masses of C and H from total g present of that substance. From the mass of C,H and O, we can c ...
First-Sample_Exam-1-Key
... problems 18-19.. Tums neutralizes stomach acid (HCl) according to the following (unbalanced) reaction: CaCO3(aq) + HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) (Hint: for the purposes of this problem, you may regard CaCO3, HCl and CaCl2 as soluble. CaCO3 is fairly soluble in acidic solutions.) Balance this ...
... problems 18-19.. Tums neutralizes stomach acid (HCl) according to the following (unbalanced) reaction: CaCO3(aq) + HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) (Hint: for the purposes of this problem, you may regard CaCO3, HCl and CaCl2 as soluble. CaCO3 is fairly soluble in acidic solutions.) Balance this ...
Oxidation of Pyrite and Marcasite by
... of bacteria depends on many physical, chemical and biological factors [1]. Its efficiency is affected by environmental conditions but also on bacteria activity and the structure of metal-bearing minerals. According to one approach explaining the role of bacteria in the process of metal leaching, the ...
... of bacteria depends on many physical, chemical and biological factors [1]. Its efficiency is affected by environmental conditions but also on bacteria activity and the structure of metal-bearing minerals. According to one approach explaining the role of bacteria in the process of metal leaching, the ...
Atoms, Molecules and Ions
... A base can be defined as a substance that yields hydroxide ions (OH-) when dissolved in water. As an ionic compound, it follows the same nomenclature, the anion is always hydroxide ...
... A base can be defined as a substance that yields hydroxide ions (OH-) when dissolved in water. As an ionic compound, it follows the same nomenclature, the anion is always hydroxide ...
Document
... ______ b (halogen) 15) The Noble Gases are a very reactive group of mostly nonmetals whose atoms gain or share one electron in chemical reactions. a) True b) False ______ b (metals) 16) Nonmetals are good conductors of heat and electricity. a) True b) False ______ b (increasing) 17) From left to rig ...
... ______ b (halogen) 15) The Noble Gases are a very reactive group of mostly nonmetals whose atoms gain or share one electron in chemical reactions. a) True b) False ______ b (metals) 16) Nonmetals are good conductors of heat and electricity. a) True b) False ______ b (increasing) 17) From left to rig ...
Acid Rain - Controlled Assessment
... Acid rain kills trees. It runs into rivers and gathers in lakes. Eventually, lakes become too acidic, and plants and fish begin to die. Acid rain reacts with limestone and damages limestone buildings.When car fuel is burnt sulphur dioxide and other various nitrogen oxides are produced. When these mi ...
... Acid rain kills trees. It runs into rivers and gathers in lakes. Eventually, lakes become too acidic, and plants and fish begin to die. Acid rain reacts with limestone and damages limestone buildings.When car fuel is burnt sulphur dioxide and other various nitrogen oxides are produced. When these mi ...
1. Cl2 + 2Br- ® 2Cl- + Br2 formulae correct for elements 1 correct
... (do not allow bonds or no forces, allow inter molecular forces are weak, do not allow they have weak forces / bonds) so little heat / energy is required before they can overcome forces / move freely / break out of solid structure / lattice (N.B. second point can be gained even if first is not) ...
... (do not allow bonds or no forces, allow inter molecular forces are weak, do not allow they have weak forces / bonds) so little heat / energy is required before they can overcome forces / move freely / break out of solid structure / lattice (N.B. second point can be gained even if first is not) ...
Final Exam - W09
... Write the possible four quantum numbers n, l, ml, and ms for each of the following electrons. ...
... Write the possible four quantum numbers n, l, ml, and ms for each of the following electrons. ...
Lab Stuff - WW-P K
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
Nugget
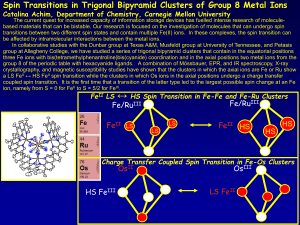
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
... transitions between two different spin states and contain multiple Fe(II) ions. In these complexes, the spin transition can be affected by intramolecular interactions between the metal ions. In collaborative studies with the Dunbar group at Texas A&M, Musfeldt group at University of Tennessee, and P ...
Answers - U of L Class Index
... Why would BeCl2 be a molecular compound when all of the other alkaline earth metals react with chlorine gas to give ionic compounds? [2 marks] The Be2+ cation is very small. Therefore, its charge density is much higher than any other M2+ cation formed from an alkaline earth metal. This makes Be2+ a ...
... Why would BeCl2 be a molecular compound when all of the other alkaline earth metals react with chlorine gas to give ionic compounds? [2 marks] The Be2+ cation is very small. Therefore, its charge density is much higher than any other M2+ cation formed from an alkaline earth metal. This makes Be2+ a ...
Chemical bond - Physical Science
... ▫ Ionic compounds are neutral because the total positive charge of the cations equals the total negative charge of the anions ...
... ▫ Ionic compounds are neutral because the total positive charge of the cations equals the total negative charge of the anions ...
SCH 3U - mquagliaoths
... 48a) You could decompose magnesium carbonate to generate magnesium oxide or you could decompose magnesium hydroxide to generate magnesium oxide. b) MgCO3(s) MgO(s) + CO2(g) or Mg(OH)2(aq) H2O(l) + MgO(aq) Pg. 145 15a) both – because the metal is burning in oxygen (combustion) and two smaller com ...
... 48a) You could decompose magnesium carbonate to generate magnesium oxide or you could decompose magnesium hydroxide to generate magnesium oxide. b) MgCO3(s) MgO(s) + CO2(g) or Mg(OH)2(aq) H2O(l) + MgO(aq) Pg. 145 15a) both – because the metal is burning in oxygen (combustion) and two smaller com ...
final exam review packet
... Be able to identify formulas as ionic or metallic Be able to draw electron dot diagrams of ions ...
... Be able to identify formulas as ionic or metallic Be able to draw electron dot diagrams of ions ...
Acid-Base Studies
... Many substances can be classified as acids or bases. There are three definitions used to describe acids and bases, but we consider only the Brønsted definition here. In this theory, an acid is a proton (H+ ) donor and acids can usually be recognized because protons that can be transferred are writte ...
... Many substances can be classified as acids or bases. There are three definitions used to describe acids and bases, but we consider only the Brønsted definition here. In this theory, an acid is a proton (H+ ) donor and acids can usually be recognized because protons that can be transferred are writte ...
Physics 213 — Problem Set 5 (Due before Feb. 26) Spring 1998
... Consider two thin, conducting, spherical shells as in Figure P25.76 of your text. The inner shell has a radius r1 = 15 cm and a charge of 10 nC. The outer shell has a radius r2 = 30 cm and a charge of −15 nC. Find (a) the electric field E and (b) the electric potential V in regions A, B, and C of th ...
... Consider two thin, conducting, spherical shells as in Figure P25.76 of your text. The inner shell has a radius r1 = 15 cm and a charge of 10 nC. The outer shell has a radius r2 = 30 cm and a charge of −15 nC. Find (a) the electric field E and (b) the electric potential V in regions A, B, and C of th ...
www.tutor-homework.com (for tutoring, homework help, or help with
... Cubane is a compound composed of only C and H. It contains 92.26% C. What is its empirical formula? a. CH b. CH2 c. C2H3 d. C2H5 e. C3H4 ...
... Cubane is a compound composed of only C and H. It contains 92.26% C. What is its empirical formula? a. CH b. CH2 c. C2H3 d. C2H5 e. C3H4 ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
Unit 8 Note Packet
... 2. Balance a chemical equation based upon the law of conservation of matter. 3. Analyze or draw a graph for the energy change of a chemical reaction. 4. Calculate the heat of solution for a given compound. We are looking for: 1a. Given the word equation/sentence, translate it into a formula chemical ...
... 2. Balance a chemical equation based upon the law of conservation of matter. 3. Analyze or draw a graph for the energy change of a chemical reaction. 4. Calculate the heat of solution for a given compound. We are looking for: 1a. Given the word equation/sentence, translate it into a formula chemical ...
Introduction to enzymes
... When the substrate concentration becomes large enough to force the equilibrium to form completely all ES the second step in the reaction becomes rate limiting because no more ES can be made and the enzyme-substrate complex is at its maximum value. ...
... When the substrate concentration becomes large enough to force the equilibrium to form completely all ES the second step in the reaction becomes rate limiting because no more ES can be made and the enzyme-substrate complex is at its maximum value. ...
Many thermal and chemical reactions occur during the roasting
... Sucrose is the principle sugar in coffee. The melting point of pure crystalline sucrose is in the 320-392 degrees F with 370 degrees F most commonly accepted. Degradation of dry sucrose can occur as low as 194 degrees F. and begins with the cleavage of the glycosidic bond followed by condensation an ...
... Sucrose is the principle sugar in coffee. The melting point of pure crystalline sucrose is in the 320-392 degrees F with 370 degrees F most commonly accepted. Degradation of dry sucrose can occur as low as 194 degrees F. and begins with the cleavage of the glycosidic bond followed by condensation an ...
Chemistry 534
... Cappuccino is a mixture of coffee and milk. The Café Entropy has determined that the best temperature for cappuccino is 45.5 C. The initial temperature of hot coffee without milk is 70.5 C. What volume of milk, at 4.0 C, must be added to 160.0 mL of hot coffee in order to obtain the desired temperat ...
... Cappuccino is a mixture of coffee and milk. The Café Entropy has determined that the best temperature for cappuccino is 45.5 C. The initial temperature of hot coffee without milk is 70.5 C. What volume of milk, at 4.0 C, must be added to 160.0 mL of hot coffee in order to obtain the desired temperat ...
chapter-2 - HCC Learning Web
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be approximated by the mass number ...
... • Atoms of the various elements differ in number of subatomic particles • An element’s atomic number is the number of protons in its nucleus • An element’s mass number is the sum of protons plus neutrons in the nucleus • Atomic mass, the atom’s total mass, can be approximated by the mass number ...
Electrochemistry
Electrochemistry is the branch of physical chemistry that studies chemical reactions which take place at the interface of an electrode, usually a solid metal or a semiconductor, and an ionic conductor, the electrolyte. These reactions involve electric charges moving between the electrodes and the electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.