Solutions
... molecules or ions. • Mixtures in chemistry are combinations of different substances where each substance retains its chemical properties. ...
... molecules or ions. • Mixtures in chemistry are combinations of different substances where each substance retains its chemical properties. ...
PREPARATORY PROBLEMS (Theoretical)
... applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device which breaks the glass with the poison when the particle enters the counter. Near the glas ...
... applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device which breaks the glass with the poison when the particle enters the counter. Near the glas ...
Worksheet Significant Figures
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
PREPARATORY PROBLEMS
... applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device which breaks the glass with the poison when the particle enters the counter. Near the glas ...
... applied to macrosystems. To illustrate this idea, E. Schrödinger proposed the following mental experiment. Consider the Geiger counter which detects the entering electrons. The counter is connected to a device which breaks the glass with the poison when the particle enters the counter. Near the glas ...
Chemistry - Set as Home Page
... The ions having the same electronic configuration are called iso electronic. ...
... The ions having the same electronic configuration are called iso electronic. ...
Band Theories
... First atom supplies an s orbital at a certain energy 2nd atom brought up overlaps with 1st atom and forms bonding and antibonding orbitals 3rd atom added overlaps it nearest neighbor (and only slightly its next nearest neighbor and three molecular orbitals result, one bonding, one antibonding and on ...
... First atom supplies an s orbital at a certain energy 2nd atom brought up overlaps with 1st atom and forms bonding and antibonding orbitals 3rd atom added overlaps it nearest neighbor (and only slightly its next nearest neighbor and three molecular orbitals result, one bonding, one antibonding and on ...
Thermochemistry Energy and Its Units
... Energy There are three broad concepts of energy: Kinetic energy is the energy associated with an object by virtue of its motion. Potential energy is the energy an object has by virtue of its position in a field of force. Internal energy is the sum of the kinetic and potential energies of the partic ...
... Energy There are three broad concepts of energy: Kinetic energy is the energy associated with an object by virtue of its motion. Potential energy is the energy an object has by virtue of its position in a field of force. Internal energy is the sum of the kinetic and potential energies of the partic ...
Week 7 - Acid-base, redox
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
... Classifying, Writing, and Balancing Redox Reactions We previously classified, wrote, and balanced precipitation, acid-base, and gas-forming reactions. Redox reactions have electron transfer, and that is what sets them apart from the other reaction types. With redox, one atom loses one or more electr ...
A2 Module 2814: Chains, Rings and Spectroscopy
... normally show a range of different oxidation states e.g. Fe2+ and Fe3+; Cu+ and Cu2+. Generally transition metals show a fairly steady increase in successive ionisation energies until all the 4s and 3d electrons have been removed, when there is a sharp jump as the closed [Ar] shell is broken into. G ...
... normally show a range of different oxidation states e.g. Fe2+ and Fe3+; Cu+ and Cu2+. Generally transition metals show a fairly steady increase in successive ionisation energies until all the 4s and 3d electrons have been removed, when there is a sharp jump as the closed [Ar] shell is broken into. G ...
S. Y. B. Sc. Chemistry
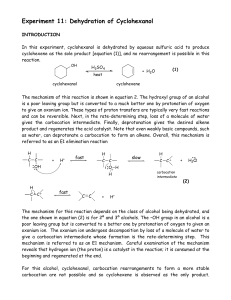
... Chapter 5 : Functional group Inter conversion i) Know different function groups ii) Know step up and step down reactions iii) Specific reagent for specific conversion iv) Able to suggest synthetic route for given target molecule v) Predict major and minor product in the given reaction if possible Ch ...
... Chapter 5 : Functional group Inter conversion i) Know different function groups ii) Know step up and step down reactions iii) Specific reagent for specific conversion iv) Able to suggest synthetic route for given target molecule v) Predict major and minor product in the given reaction if possible Ch ...