United States Patent
... in the IS-position. Osuka et al .• "Synthesis of Benzochlorin Monomer. Dimer, and Porphyrin-Benzochlorin Heterodimer from 5-Aryl- and 5J5-Diaryloctaethylporphyrins," Bull. Chem. Soc. lpn., 65.3322-30 (1992). Some of these derivatives have shown strong absorptions in the visible region around 700 run ...
... in the IS-position. Osuka et al .• "Synthesis of Benzochlorin Monomer. Dimer, and Porphyrin-Benzochlorin Heterodimer from 5-Aryl- and 5J5-Diaryloctaethylporphyrins," Bull. Chem. Soc. lpn., 65.3322-30 (1992). Some of these derivatives have shown strong absorptions in the visible region around 700 run ...
Substitution Rxns-a-Sn2-12-quesx
... to the one that bears the leaving group also slows the rate of bimolecular nucleophilic substitution, but the effect is smaller. ...
... to the one that bears the leaving group also slows the rate of bimolecular nucleophilic substitution, but the effect is smaller. ...
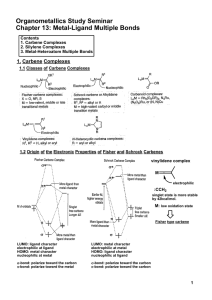
Metal-Ligand Multiple Bonds
... (Schrock-type carbene complexes also undergo the [2+2] reaction.) ...
... (Schrock-type carbene complexes also undergo the [2+2] reaction.) ...
Mechanistic notation
... alkyloxonium ion (2) bimolecular displacement of water from alkyloxonium ion by halide ...
... alkyloxonium ion (2) bimolecular displacement of water from alkyloxonium ion by halide ...
Eleanor Roosevelt High School Dimitri Saliani, Principal Angela
... bonding? How can we predict whether a bond will be ionic or covalent? What is a dipole? What is a hydrogen bond? How do we compare/contrast metallic, ionic, and covalent bonds? How is the polarity of a molecule related to its symmetry? What are intermolecular forces and what are the diff ...
... bonding? How can we predict whether a bond will be ionic or covalent? What is a dipole? What is a hydrogen bond? How do we compare/contrast metallic, ionic, and covalent bonds? How is the polarity of a molecule related to its symmetry? What are intermolecular forces and what are the diff ...
Chapter 7: Recent advances in enzyme technology
... the water activity in these systems they can be used to transfer to other acceptors. Examples of this can be found in the transesterification reactions of esterases and lipases, described more fully later, and the (undesirable) formation of isomaltose from glucose catalysed by glucoamylase. Restrict ...
... the water activity in these systems they can be used to transfer to other acceptors. Examples of this can be found in the transesterification reactions of esterases and lipases, described more fully later, and the (undesirable) formation of isomaltose from glucose catalysed by glucoamylase. Restrict ...
A model based on equations of kinetics to study nitrogen dioxide
... Simulation model In a comprehensive and complete model to study the conversion of the nitrogen dioxide molecules and their temporal behavior within the plasma discharge reactor, the following phenomena should be accounted for: (a) the temporal variations of the different charged species and their co ...
... Simulation model In a comprehensive and complete model to study the conversion of the nitrogen dioxide molecules and their temporal behavior within the plasma discharge reactor, the following phenomena should be accounted for: (a) the temporal variations of the different charged species and their co ...
Chemical reaction model:
... The process of natural oxidative degradation takes years and it is not possible to wait that long for data to be available when testing new materials. Hence, the oxidation of polymer is frequently carried at elevated temperature and pressure of oxygen. Elevation in temperature and pressure leads to ...
... The process of natural oxidative degradation takes years and it is not possible to wait that long for data to be available when testing new materials. Hence, the oxidation of polymer is frequently carried at elevated temperature and pressure of oxygen. Elevation in temperature and pressure leads to ...
17.2.3 Interhalogen compounds(65-67)
... from the melt and features zigzag chains of molecules (Fig. 17.6) with two different IC1 units and appreciable interchain intermolecular bonding. The packing is somewhat different in the yellow, metastable (B) form (Fig. 17.6) which can be obtained as brownish-red crystals from strongly supercooled ...
... from the melt and features zigzag chains of molecules (Fig. 17.6) with two different IC1 units and appreciable interchain intermolecular bonding. The packing is somewhat different in the yellow, metastable (B) form (Fig. 17.6) which can be obtained as brownish-red crystals from strongly supercooled ...
Enantioselective one-pot synthesis of dihydroquinolones via BINOL
... markers are lost in the facile epimerization of the product.26 ...
... markers are lost in the facile epimerization of the product.26 ...
thermodynamics
... 27. Given that ∆H = 0 for mixing of two gases. Explain whether the diffusion of these gases into each other in a closed container is a spontaneous process or not? 28. Heat has randomising influence on a system and temperature is the measure of average chaotic motion of particles in the system. Write ...
... 27. Given that ∆H = 0 for mixing of two gases. Explain whether the diffusion of these gases into each other in a closed container is a spontaneous process or not? 28. Heat has randomising influence on a system and temperature is the measure of average chaotic motion of particles in the system. Write ...