Lessons 9
... Surroundings: All matter around the system that is capable of absorbing or releasing thermal energy. Consider the following reaction taking place in your body cells: C6H12O6 + 6O2 Æ 6H2O +2CO2 + energy The molecules (glucose, oxygen, water, and carbon dioxide are the chemical system, while the surro ...
... Surroundings: All matter around the system that is capable of absorbing or releasing thermal energy. Consider the following reaction taking place in your body cells: C6H12O6 + 6O2 Æ 6H2O +2CO2 + energy The molecules (glucose, oxygen, water, and carbon dioxide are the chemical system, while the surro ...
Addition Reactions of Carbonyls Part 1
... Addition of hydride to a carbonyl group (reducing it to an alcohol) is also a very useful reaction. Hydride is H-; however, we cannot just add H- to C=O as H- is much more basic than nucleophilic (giving the enolate where possible). NaH and KH are great bases! So, how can we make an electron rich, n ...
... Addition of hydride to a carbonyl group (reducing it to an alcohol) is also a very useful reaction. Hydride is H-; however, we cannot just add H- to C=O as H- is much more basic than nucleophilic (giving the enolate where possible). NaH and KH are great bases! So, how can we make an electron rich, n ...
Homo-coupling of terminal alkynes on a noble metal surface
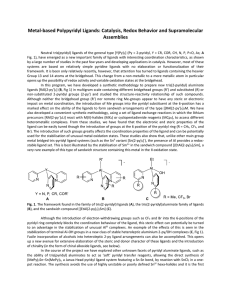
... image shows a smooth connexion between the two TEB moieties, which has an apparent height slightly lower than the two benzene rings. For comparison, the Ag bis-acetylide complex, further referred to as organometallic dimer, would exhibit a characteristic substructure in the connecting waist originat ...
... image shows a smooth connexion between the two TEB moieties, which has an apparent height slightly lower than the two benzene rings. For comparison, the Ag bis-acetylide complex, further referred to as organometallic dimer, would exhibit a characteristic substructure in the connecting waist originat ...
In Class Problems and Notes AP Chemistry General Equilibrium
... (c) Write the equilibrium expression for the equilibrium constant, KP, and calculate its value for the reaction under the conditions in (a). (d) If 110. grams of solid NaHCO3 had been placed in the 5.00-liter container and heated to 160ºC, what would the total pressure have been at equilibrium? Expl ...
... (c) Write the equilibrium expression for the equilibrium constant, KP, and calculate its value for the reaction under the conditions in (a). (d) If 110. grams of solid NaHCO3 had been placed in the 5.00-liter container and heated to 160ºC, what would the total pressure have been at equilibrium? Expl ...
1 Course Code– CH1141 Semester – I Credit
... 1. The angular momentum of the electron according to the Bohr model is an integral multiple of ……… 2. Write Rydberg equation. 3. State first law of thermodynamics 4. What is Gibbs energy? 5. What is isothermal expansion? 6. What is the thermodynamic criteria of a spontaneous process? 7. Why BCl3 is ...
... 1. The angular momentum of the electron according to the Bohr model is an integral multiple of ……… 2. Write Rydberg equation. 3. State first law of thermodynamics 4. What is Gibbs energy? 5. What is isothermal expansion? 6. What is the thermodynamic criteria of a spontaneous process? 7. Why BCl3 is ...
Physical Chemistry Problems. ©Mike Lyons 2009
... a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed by the system. Hence derive a relationship between the change in ...
... a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed by the system. Hence derive a relationship between the change in ...
chapter 2. electrochemical methods and materials 17
... limiting current for the re-oxidation of R occurs, not all of the reduced form produced at the disc will reach the ring surface. For a given system, the fraction of R collected will depend on the geometry of the disc and ring only. The value of N is given by the Albery-Bruckenstein equation (see [72 ...
... limiting current for the re-oxidation of R occurs, not all of the reduced form produced at the disc will reach the ring surface. For a given system, the fraction of R collected will depend on the geometry of the disc and ring only. The value of N is given by the Albery-Bruckenstein equation (see [72 ...
Thermodynamics: the Second Law
... rest with all its energy degraded into the thermal motion of floor atoms. A ball resting on a warm floor has never been observed to start bouncing, something special need to happen. Some of the thermal motion of the atoms in the floor would have to accumulate in a single small object, the ball. This ...
... rest with all its energy degraded into the thermal motion of floor atoms. A ball resting on a warm floor has never been observed to start bouncing, something special need to happen. Some of the thermal motion of the atoms in the floor would have to accumulate in a single small object, the ball. This ...
IB Chemistry HL Topic5 Questions 1. Which combination of ionic
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. ...
... The lattice enthalpy of an ionic compound can be calculated using a Born-Haber cycle. Using lithium fluoride as the example, construct a Born-Haber cycle, labelling the cycle with the formulas and state symbols of the species present at each stage. ...
Audit Schedule
... and Kelvin. 5. To apply the rules for significant figures. 6. To distinguish between precision and accuracy. Study Hints: 1. In the textbook, the problems are worked with a calculator and the answer is rounded to the correct number of significant figures only at the end of the problem. Rounding off ...
... and Kelvin. 5. To apply the rules for significant figures. 6. To distinguish between precision and accuracy. Study Hints: 1. In the textbook, the problems are worked with a calculator and the answer is rounded to the correct number of significant figures only at the end of the problem. Rounding off ...